Acetyl-CoA: Difference between revisions
Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:Wi |
Citation bot (talk | contribs) Altered doi-broken-date. | Use this bot. Report bugs. | #UCB_CommandLine |
||
| (271 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
{{cs1 config|name-list-style=vanc}} |
|||
{{chembox |
|||
{{Multiple issues|{{citation style|details=Multiple page-numbers in a single ref that is used multiple times: unclear which supports which<!--(use {{tl|rp}}?)-->. Some ISBN might be for wrong edition of the book. Need page-numbers for refs to whole broad-coverage textbooks. |date=August 2017}} |
|||
| verifiedrevid = 443366059 |
|||
{{Expert needed|Biochemistry |
|||
| ImageFile = Acetyl-CoA-2D.svg |
|||
| date = August 2024 |
|||
| reason = Per [[Talk:Fatty acyl-CoA esters#c-Teaktl17-20240804223600-DeemDeem52-20240529152800|this talk page]]: Needs more context about benzoyl-CoA and lactoyl-CoA. Some content needs to be moved to acyl-CoA article to fatty acyl-CoA. |
|||
}}}}{{chembox |
|||
| Verifiedfields = changed |
|||
| Watchedfields = changed |
|||
| verifiedrevid = 477240040 |
|||
| Name = Acetyl-CoA |
|||
| ImageFile = Acetyl-CoA-2D_colored.svg |
|||
| ImageSize = 320 |
| ImageSize = 320 |
||
| ImageFile2 = Acetyl-CoA-3D-balls.png |
| ImageFile2 = Acetyl-CoA-3D-balls.png |
||
| ImageSize2 = 320 |
| ImageSize2 = 320 |
||
| ImageFile3 = Acetyl-CoA-3D-vdW.png |
|||
| IUPACName = |
|||
| |
| ImageSize3 = 320 |
||
| PIN = ''O''<sup>1</sup>-<nowiki/>{(3''R'')-4-[(3-<nowiki/>{[2-(Acetylsulfanyl)ethyl]amino}-3-oxopropyl)amino]-3-hydroxy-2,2-dimethyl-4-oxobutyl} ''O''<sup>3</sup>-<nowiki/>{[(2''R'',3''S'',4''R'',5''R'')-5-(6-amino-9''H''-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methyl} dihydrogen diphosphate |
|||
| OtherNames = |
|||
| Section1 = {{Chembox Identifiers |
| Section1 = {{Chembox Identifiers |
||
| IUPHAR_ligand = 3038 |
|||
| InChIKey = ZSLZBFCDCINBPY-ZSJPKINUBJ |
| InChIKey = ZSLZBFCDCINBPY-ZSJPKINUBJ |
||
| InChI = 1/C23H38N7O17P3S/c1-12(31)51-7-6-25-14(32)4-5-26-21(35)18(34)23(2,3)9-44-50(41,42)47-49(39,40)43-8-13-17(46-48(36,37)38)16(33)22(45-13)30-11-29-15-19(24)27-10-28-20(15)30/h10-11,13,16-18,22,33-34H,4-9H2,1-3H3,(H,25,32)(H,26,35)(H,39,40)(H,41,42)(H2,24,27,28)(H2,36,37,38)/t13-,16-,17-,18+,22-/m1/s1 |
| InChI = 1/C23H38N7O17P3S/c1-12(31)51-7-6-25-14(32)4-5-26-21(35)18(34)23(2,3)9-44-50(41,42)47-49(39,40)43-8-13-17(46-48(36,37)38)16(33)22(45-13)30-11-29-15-19(24)27-10-28-20(15)30/h10-11,13,16-18,22,33-34H,4-9H2,1-3H3,(H,25,32)(H,26,35)(H,39,40)(H,41,42)(H2,24,27,28)(H2,36,37,38)/t13-,16-,17-,18+,22-/m1/s1 |
||
| Line 16: | Line 27: | ||
| StdInChIKey = ZSLZBFCDCINBPY-ZSJPKINUSA-N |
| StdInChIKey = ZSLZBFCDCINBPY-ZSJPKINUSA-N |
||
| CASNo = 72-89-9 |
| CASNo = 72-89-9 |
||
| |
| CASNo_Ref = {{cascite|correct|CAS}} |
||
| CASNo_Comment = (free acid) |
|||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| UNII = 76Q83YLO3O |
|||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
||
| ChemSpiderID=392413 |
| ChemSpiderID=392413 |
||
| PubChem = 444493 |
| PubChem = 444493 |
||
| KEGG_Ref = {{keggcite|changed|kegg}} |
|||
| KEGG = C00024 |
|||
| ChEBI_Ref = {{ebicite|correct|EBI}} |
| ChEBI_Ref = {{ebicite|correct|EBI}} |
||
| ChEBI = 15351 |
| ChEBI = 15351 |
||
| Line 26: | Line 42: | ||
}} |
}} |
||
| Section2 = {{Chembox Properties |
| Section2 = {{Chembox Properties |
||
| C=23 | H=38 | N=7 | O=17 | P=3 | S=1 |
|||
| Formula = C<sub>23</sub>H<sub>38</sub>N<sub>7</sub>O<sub>17</sub>P<sub>3</sub>S |
|||
| MolarMass = 809.57 g/mol |
|||
| Appearance = |
| Appearance = |
||
| Density = |
| Density = |
||
| MeltingPt = |
| MeltingPt = |
||
| BoilingPt = |
| BoilingPt = |
||
| LambdaMax = 260 nm; 232 nm<ref name="lamda_source_1">{{cite book |last1=Dawson |first1=Rex M. C. |last2=Elliott |first2=Daphne C. |last3=Elliott |first3=William H. |last4=Jones |first4=Kenneth M. |title=Data for Biochemical Research |date=2002 |publisher=Clarendon Press |isbn=978-0-19-855299-4 |edition=3rd|page=117}}</ref> |
|||
| Absorbance = [[Molar attenuation coefficient|ε<sub>260</sub>]] = 16.4 mM<sup>−1</sup> cm<sup>−1</sup> (adenosine)<ref name="lamda_source_1" /><br /> [[Molar attenuation coefficient|ε<sub>232</sub>]] = 8.7 mM<sup>−1</sup> cm<sup>−1</sup> (thioester)<ref name="lamda_source_1" /><br /> Δ[[Molar attenuation coefficient|ε<sub>232</sub>]] on thioester hydrolysis = −4.5 mM<sup>−1</sup> cm<sup>−1</sup><ref name="lamda_source_1" /> |
|||
}} |
}} |
||
| Section3 = {{Chembox Hazards |
| Section3 = {{Chembox Hazards |
||
| Solubility = |
|||
| MainHazards = |
| MainHazards = |
||
| FlashPt = |
| FlashPt = |
||
| |
| AutoignitionPt = |
||
}} |
}} |
||
| Section4 = |
|||
| Section5 = |
|||
| Section6 = |
|||
}} |
}} |
||
'''Acetyl-CoA''' ('''acetyl coenzyme A''') is a molecule that participates in many [[biochemical reaction]]s in protein, carbohydrate and lipid [[metabolism]].<ref>{{cite web|url=http://chemistry.elmhurst.edu/vchembook/623acetylCoAfate.html|title=Acetyl CoA Crossroads|website=chemistry.elmhurst.edu|access-date=2016-11-08|archive-date=2016-11-15|archive-url=https://web.archive.org/web/20161115202146/http://chemistry.elmhurst.edu/vchembook/623acetylCoAfate.html|url-status=dead}}</ref> Its main function is to deliver the [[acetyl]] group to the [[citric acid cycle]] (Krebs cycle) to be [[oxidation|oxidized]] for energy production. |
|||
'''Acetyl coenzyme A''' or '''acetyl-CoA''' is an important molecule in [[metabolism]], used in many [[biochemical reaction]]s. Its main function is to convey the [[carbon]] [[atom]]s within the [[acetyl]] group to the [[citric acid cycle]] to be [[oxidation|oxidized]] for energy production. In chemical structure, acetyl-CoA is the [[thioester]] between [[coenzyme A]] (a [[thiol]]) and [[acetic acid]] (an [[acyl]] group carrier). Acetyl-CoA is produced during the second step of aerobic [[cellular respiration]], [[pyruvate decarboxylation]], which occurs in the [[Matrix (biology)|matrix]] of the [[mitochondria]]. Acetyl-CoA then enters the citric acid cycle. |
|||
[[Coenzyme A]] (CoASH or CoA) consists of a [[cysteamine|β-mercaptoethylamine group]] linked to [[pantothenic acid]] (vitamin B5) through an [[amide linkage]]<ref>{{cite web|url=http://library.med.utah.edu/NetBiochem/FattyAcids/2_4.html|title=Fatty Acids -- Structure of Acetyl CoA|website=library.med.utah.edu|access-date=2017-06-02}}</ref> and 3'-phosphorylated ADP. The acetyl group (indicated in blue in the structural diagram on the right) of acetyl-CoA is linked to the [[sulfhydryl]] substituent of the β-mercaptoethylamine group. This [[thioester]] linkage is a "high energy" bond, which is particularly reactive. [[Hydrolysis]] of the thioester bond is [[exergonic]] (−31.5 kJ/mol). |
|||
Acetyl-CoA is also an important component in the biogenic synthesis of the neurotransmitter [[acetylcholine]]. [[Choline]], in combination with acetyl-CoA, is catalyzed by the enzyme [[choline acetyltransferase]] to produce acetylcholine and a [[coenzyme a]] byproduct. |
|||
CoA is acetylated to acetyl-CoA by the breakdown of [[carbohydrates]] through [[glycolysis]] and by the breakdown of [[fatty acids]] through [[Beta oxidation|β-oxidation]]. Acetyl-CoA then enters the citric acid cycle, where the acetyl group is oxidized to carbon dioxide and water, and the energy released is captured in the form of 11 [[Adenosine triphosphate|ATP]] and one [[Guanosine triphosphate|GTP]] per acetyl group. |
|||
==Functions== |
|||
===Pyruvate dehydrogenase and pyruvate formate lyase reactions=== |
|||
The oxidative conversion of [[pyruvate]] into acetyl-CoA is referred to as the '''pyruvate dehydrogenase reaction'''. It is catalyzed by the [[pyruvate dehydrogenase complex]]. Other conversions between pyruvate and acetyl-CoA are possible. For example, [[pyruvate formate lyase]] disproportionates pyruvate into acetyl-CoA and [[formic acid]]. |
|||
[[Konrad Bloch]] and [[Feodor Lynen]] were awarded the 1964 [[Nobel Prize in Physiology or Medicine]] for their discoveries linking acetyl-CoA and fatty acid metabolism. [[Fritz Lipmann]] won the Nobel Prize in 1953 for his discovery of the cofactor [[coenzyme A]].<ref>{{cite web |title=All Nobel Prizes in Physiology or Medicine |url=https://www.nobelprize.org/prizes/lists/all-nobel-laureates-in-physiology-or-medicine/ |website=The Nobel Prize}}</ref> |
|||
===Fatty acid metabolism=== |
|||
In animals, acetyl-CoA is essential to the balance between [[carbohydrate metabolism]] and [[fat]] metabolism (see [[fatty acid synthesis]]). In normal circumstances, acetyl-CoA from fatty acid metabolism feeds into the [[citric acid cycle]], contributing to the cell's energy supply. In the [[liver]], when levels of circulating fatty acids are high, the production of acetyl-CoA from fat breakdown exceeds the cellular energy requirements. To make use of the energy available from the excess acetyl-CoA, [[ketone bodies]] are produced, which can then circulate in the blood. |
|||
== Role == |
|||
In some circumstances, this can lead to the presence of very high levels of ketone bodies in the [[blood]], a condition called [[ketosis]]. Benign dietary ketosis can safely occur in people following [[low-carbohydrate diet]]s, which cause fats to be metabolised as a major source of energy. This is different from ketosis brought on as a result of starvation, and from [[ketoacidosis]], a dangerous condition that can affect [[diabetics]]. |
|||
Acetyl-CoA is a [[metabolic intermediate]] that is involved in many metabolic pathways in an organism. It is produced during the breakdown of [[glucose]], [[fatty acids]], and [[Amino acid|amino acids]], and is used in the synthesis of many other [[biomolecules]], including [[cholesterol]], [[fatty acid]]s, and [[ketone bodies]]. Acetyl-CoA is also a key molecule in the [[citric acid cycle]], which is a series of chemical reactions that occur in the [[Mitochondrion|mitochondria]] of cells and is responsible for generating energy in the form of [[Adenosine triphosphate|ATP]].<ref name="pmid31387584">{{cite journal |vauthors=Zhang S, Yang W, Chen H, Liu B, Lin B, Tao Y |title=Metabolic engineering for efficient supply of acetyl-CoA from different carbon sources in Escherichia coli |journal=Microb Cell Fact |volume=18 |issue=1 |pages=130 |date=August 2019 |pmid=31387584 |doi=10.1186/s12934-019-1177-y |pmc=6685171 |url= |doi-access=free }}</ref><ref>{{cite web | url=https://bio.libretexts.org/Bookshelves/Microbiology/Microbiology_%28Boundless%29/05%3A_Microbial_Metabolism/5.12%3A_Biosynthesis/5.12G%3A_The_Acetyl-CoA_Pathway | title=5.12G: The Acetyl-CoA Pathway | date=9 May 2017 }}</ref> |
|||
In addition, acetyl-CoA is a precursor for the biosynthesis of various acetyl-chemicals, acting as an intermediate to transfer an acetyl group during the biosynthesis of those acetyl-chemicals. Acetyl-CoA is also involved in the regulation of various cellular mechanisms by providing acetyl groups to target amino acid residues for post-translational [[acetylation]] reactions of proteins. |
|||
In plants, ''de novo'' fatty acid synthesis occurs in the plastids. Many seeds accumulate large reservoirs of seed oils to support [[germination]] and early growth of the seedling before it is a net photosynthetic organism. Fatty acids are incorporated into membrane lipids, the major component of most membranes. |
|||
== |
== Biosynthesis == |
||
The acetylation of CoA is determined by the carbon sources.<ref>{{cite journal|last1=Hynes|first1=Michael J.|last2=Murray|first2=Sandra L.|date=2010-07-01|title=ATP-Citrate Lyase Is Required for Production of Cytosolic Acetyl Coenzyme A and Development in Aspergillus nidulans|journal=Eukaryotic Cell|language=en|volume=9|issue=7|pages=1039–1048|doi=10.1128/EC.00080-10|issn=1535-9778|pmc=2901662|pmid=20495057}}</ref><ref>{{cite journal|last1=Wellen|first1=Kathryn E.|last2=Thompson|first2=Craig B.|date=2012-04-01|title=A two-way street: reciprocal regulation of metabolism and signalling|journal=Nature Reviews Molecular Cell Biology|language=en|volume=13|issue=4|pages=270–276|doi=10.1038/nrm3305|issn=1471-0072|pmid=22395772|s2cid=244613}}</ref> |
|||
* Two acetyl-CoA molecules can be [[Condensation reaction|condensed]] to create [[acetoacetyl-CoA]], the first step in the HMG-CoA/[[mevalonic acid pathway]], leading to synthesis of [[isoprenoid]]s. In animals, HMG-CoA is a vital precursor to [[cholesterol]] and [[Ketone bodies|ketone body]] synthesis. |
|||
* Acetyl-CoA is also the source of the acetyl group incorporated onto certain lysine residues of histone and nonhistone proteins in the posttranslational modification [[acetylation]], a reaction catalyzed by [[acetyltransferases]]. |
|||
* In plants and animals, cytosolic acetyl-CoA is synthesized by [[ATP citrate lyase]].<ref>{{cite journal | last1 = Fatland | first1 = B. L. | last2 = Ke | first2 = J | last3 = Anderson | first3 = MD | last4 = Mentzen | first4 = WI | last5 = Cui | first5 = LW | last6 = Allred | first6 = CC | last7 = Johnston | first7 = JL | last8 = Nikolau | first8 = BJ | last9 = Wurtele | first9 = ES | title = Molecular Characterization of a Heteromeric ATP-Citrate Lyase That Generates Cytosolic Acetyl-Coenzyme a in Arabidopsis | journal = Plant Physiology | volume = 130 | issue = 2 | pages = 740 | year = 2002 | pmid = 12376641 | pmc = 166603 | doi = 10.1104/pp.008110}}</ref> When glucose is abundant in the blood of animals, it is converted via [[glycolysis]] in the [[cytosol]] to [[pyruvate]], and then to acetyl-CoA in the mitochondrion. The excess of acetyl-CoA results in production of excess [[citrate]], which is exported into the cytosol to give rise to cytosolic acetyl-CoA. |
|||
* Acetyl-CoA can be [[carboxylated]] in the cytosol by [[acetyl-CoA carboxylase]], giving rise to [[malonyl-CoA]], a substrate required for synthesis of flavonoids and related polyketides, for elongation of fatty acids to produce waxes, [[cuticle]], and seed oils in members of the [[Brassica]] family, and for malonation of proteins and other phytochemicals. <ref>{{cite journal | last1 = Fatland | first1 = B. L. | title = Reverse Genetic Characterization of Cytosolic Acetyl-CoA Generation by ATP-Citrate Lyase in Arabidopsis | journal = The Plant Cell Online | volume = 17 | pages = 182 | year = 2005 | doi = 10.1105/tpc.104.026211}}</ref> |
|||
* In plants, these include [[sesquiterpene]]s, [[brassinosteroid]]s (hormones), and membrane [[sterol]]s. |
|||
=== Extramitochondrial === |
|||
== Interactive pathway map == |
|||
* At high [[glucose]] levels, [[glycolysis]] takes place rapidly, thus increasing the amount of [[citrate]] produced from the [[citric acid cycle]]. This citrate is then exported to other [[organelle]]s outside the mitochondria to be broken into acetyl-CoA and [[oxaloacetate]] by the [[enzyme]] [[ATP citrate lyase]] (ACL). This principal reaction is coupled with the [[hydrolysis]] of ATP.<ref>{{cite book|url=https://books.google.com/books?id=d1nu4vcml8sC&q=reaction+of+ATP+citrate+lyase+produces+acetyl+coA&pg=PA253|title=Functional Metabolism: Regulation and Adaptation|last=Storey|first=Kenneth B.|date=2005-02-25|publisher=John Wiley & Sons|isbn=9780471675570|language=en}}</ref><ref>{{cite web|url=https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=ShowDetailView&TermToSearch=47|title=ACLY ATP citrate lyase [Homo sapiens (human)] - Gene - NCBI|website=www.ncbi.nlm.nih.gov|access-date=2016-11-06}}</ref> |
|||
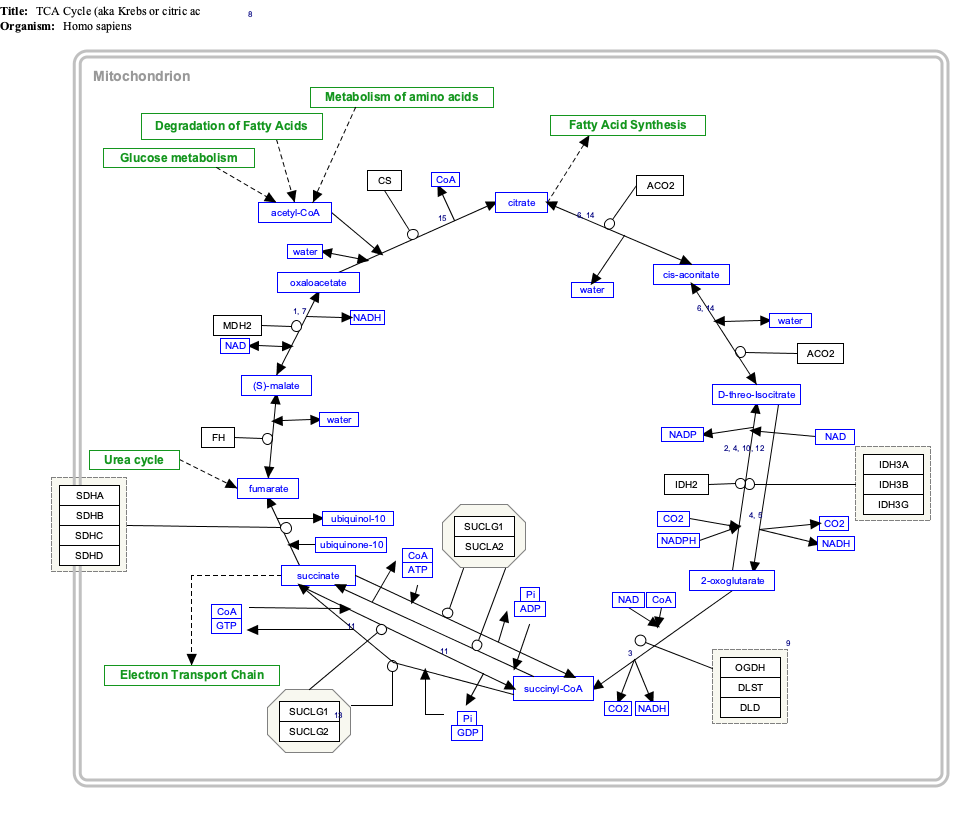
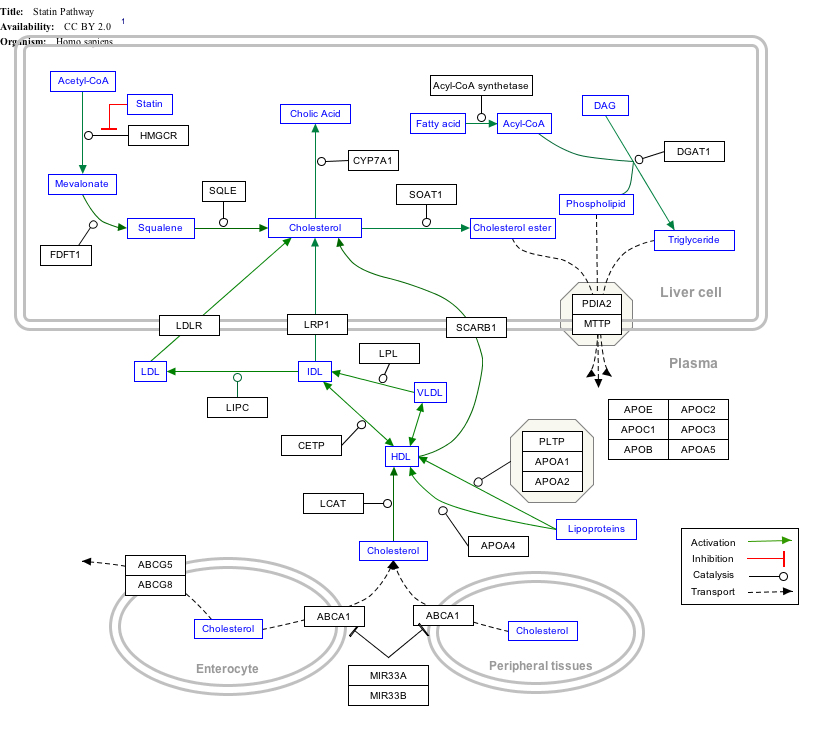
''Click on genes, proteins and metabolites below to visit [[Portal:Gene_Wiki|Gene Wiki]] pages and related Wikipedia articles. The pathway can be downloaded and edited at [http://www.wikipathways.org WikiPathways].'' |
|||
* At low glucose levels: |
|||
{| |
|||
** CoA is acetylated using [[acetate]] by [[acetyl-CoA synthetase]] (ACS), also coupled with [[adenosine triphosphate|ATP]] hydrolysis.<ref>{{cite journal|last=Ragsdale|first=S. W.|title=Life with carbon monoxide|journal=CRC Critical Reviews in Biochemistry and Molecular Biology|date=2004|volume=39|issue=3|pages=165–195|doi=10.1080/10409230490496577|pmid=15596550|s2cid=16194968}}</ref> |
|||
|width="390px"|{{TCACycle_WP78|highlight=Acetyl-CoA|header=}} |
|||
** [[Ethanol]] also serves as a carbon source for acetylation of CoA utilizing the enzyme [[alcohol dehydrogenase]].<ref>{{cite book|url=https://books.google.com/books?id=xN0YYypnZVkC&q=reaction+of+Acetyl+CoA+synthase+produce+Acetyl+CoA&pg=PA275|title=Textbook of Biochemistry for Dental/Nursing/Pharmacy Students|last=Chatterjea|date=2004-01-01|publisher=Jaypee Brothers Publishers|isbn=9788180612046|language=en}}{{Dead link|date=February 2024 |bot=InternetArchiveBot |fix-attempted=yes }}</ref> |
|||
|width="390px"|{{StatinPathway_WP430|highlight=Acetyl-CoA|header=}} |
|||
** Degradation of branched-chain [[ketogenic]] [[amino acid]]s such as [[valine]], [[leucine]], and [[isoleucine]] occurs. These amino acids are converted to α-ketoacids by [[transamination]] and eventually to [[isovaleryl-CoA]] through oxidative decarboxylation by an α-ketoacid dehydrogenase complex. Isovaleryl-CoA undergoes [[dehydrogenation]], [[carboxylation]] and hydration to form another CoA-derivative intermediate before it is cleaved into acetyl-CoA and [[acetoacetate]].<ref name=":0">{{cite book|url=https://archive.org/details/biochemistrychap00jere|title=Biochemistry|last1=Berg|first1=Jeremy M.|last2=Tymoczko|first2=John L.|last3=Stryer|first3=Lubert|year=2002|publisher=W. H. Freeman|isbn=978-0716730514|edition=5th}}</ref>{{page needed|date=August 2017}} |
|||
|} |
|||
=== Intramitochondrial === |
|||
[[File:Pyruvate dehydrogenase complex reaction.svg|left|thumb|[[Pyruvate dehydrogenase]] complex reaction]] |
|||
* At high glucose levels, acetyl-CoA is produced through [[glycolysis]].<ref>{{cite book|url=https://books.google.com/books?id=y8JQAwAAQBAJ&q=acetyl+coA+pathway&pg=PA149|title=Guide to Biochemistry|last=Blackstock|first=James C.|date=2014-06-28|publisher=Butterworth-Heinemann|isbn=9781483183671|language=en}}</ref> [[Pyruvate]] undergoes oxidative decarboxylation in which it loses its [[carboxyl]] group (as [[carbon dioxide]]) to form acetyl-CoA, giving off 33.5 kJ/mol of energy. The oxidative conversion of pyruvate into acetyl-CoA is referred to as the '''pyruvate dehydrogenase reaction'''. It is catalyzed by the [[pyruvate dehydrogenase complex]]. Other conversions between pyruvate and acetyl-CoA are possible. For example, [[pyruvate formate lyase]] [[disproportionates]] pyruvate into acetyl-CoA and [[formic acid]]. |
|||
[[File:Metabolism4.jpg|right|thumb|282px|[[beta-oxidation|β-Oxidation]] of [[fatty acid]]s]] |
|||
* At low glucose levels, the production of acetyl-CoA is linked to [[beta oxidation|β-oxidation]] of [[fatty acid]]s. Fatty acids are first converted to acyl-CoA. Acyl-CoA is then degraded in a four-step cycle of [[oxidation]], [[Hydration reaction|hydration]], [[oxidation]] and [[thiolysis]] catalyzed by four respective enzymes, namely [[acyl-CoA dehydrogenase]], [[enoyl-CoA hydratase]], [[3-hydroxyacyl-CoA dehydrogenase]], and [[thiolase]]. The cycle produces a new fatty acid chain with two fewer carbons and acetyl-CoA as a byproduct.<ref>{{cite journal|last1=Houten|first1=Sander Michel|last2=Wanders|first2=Ronald J. A.|date=2010-03-02|title=A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation|journal=Journal of Inherited Metabolic Disease|language=en|volume=33|issue=5|pages=469–477|doi=10.1007/s10545-010-9061-2|issn=0141-8955|pmc=2950079|pmid=20195903}}</ref> |
|||
==Functions== |
|||
=== Intermediates in various pathways === |
|||
== See also == |
|||
* [[ |
* In [[cellular respiration]] |
||
* [[ |
* [[Citric acid cycle]]: |
||
** Through a series of chemical reactions, stored energy is released through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins into [[adenosine triphosphate]] (ATP) and [[carbon dioxide]]. |
|||
* [[Fatty acid metabolism]] |
* [[Fatty acid metabolism]] |
||
** Acetyl-CoA is produced by the breakdown of both [[carbohydrate]]s (by [[glycolysis]]) and [[lipids]] (by [[Beta oxidation|β-oxidation]]). It then enters the citric acid cycle in the mitochondrion by combining with [[Oxaloacetic acid|oxaloacetate]] to form [[citric acid|citrate]].<ref name="stryer2">{{cite book |last1= Stryer |first1= Lubert | title=Biochemistry. | edition= Fourth |location= New York |publisher= W.H. Freeman and Company|date= 1995 |pages= 510–515, 559–565, 581–613, 614–623, 775–778 |isbn= 978-0-7167-2009-6 }}</ref><ref name="oxidation_of_fats">{{cite web|url=http://pharmaxchange.info/press/2013/10/oxidation-of-fatty-acids/|title=Oxidation of fatty acids|date=2013-10-11}}</ref> |
|||
* [[Acyl CoA]] |
|||
** Two acetyl-CoA molecules condense to form [[acetoacetyl-CoA]], which gives rise to the formation of [[Acetoacetic acid|acetoacetate]] and [[beta-Hydroxybutyric acid|β-hydroxybutyrate]].<ref name="stryer2" /> Acetoacetate, β-hydroxybutyrate, and their spontaneous breakdown product [[acetone]]<ref>{{cite web|url=http://watcut.uwaterloo.ca/webnotes/Metabolism/fatKetoneBodyMetabolism.html|title=Ketone body metabolism|publisher=University of Waterloo}}</ref> are frequently, but confusingly, known as [[ketone bodies]] (as they are not "bodies" at all, but water-soluble chemical substances). The ketone bodies are released by the [[liver]] into the blood. All cells with mitochondria can take ketone bodies up from the blood and reconvert them into acetyl-CoA, which can then be used as fuel in their citric acid cycles, as no other tissue can divert its oxaloacetate into the [[gluconeogenesis|gluconeogenic pathway]] in the way that the liver does. Unlike free fatty acids, ketone bodies can cross the [[blood–brain barrier]] and are therefore available as fuel for the cells of the [[central nervous system]], acting as a substitute for glucose, on which these cells normally survive.<ref name="stryer2" /> The occurrence of high levels of ketone bodies in the blood during [[starvation]], a [[low-carbohydrate diet]], prolonged heavy exercise, and uncontrolled [[Type 1 diabetes|type-1 diabetes mellitus]] is known as [[ketosis]], and in its extreme form in out-of-control type-1 diabetes mellitus, as [[ketoacidosis]]. |
|||
* [[Acetyl Co-A synthetase]] |
|||
** On the other hand, when the [[insulin]] concentration in the blood is high, and that of [[glucagon]] is low (i.e. after meals), the acetyl-CoA produced by glycolysis condenses as normal with oxaloacetate to form citrate in the mitochondrion. However, instead of continuing through the citric acid cycle to be converted to carbon dioxide and water, the citrate is removed from the mitochondrion into the [[cytoplasm]].<ref name="stryer2" /> There it is cleaved by [[ATP citrate lyase]] into acetyl-CoA and oxaloacetate. The oxaloacetate is returned to the mitochondrion as malate (and then converted back into oxaloacetate to transfer more acetyl-CoA out of the mitochondrion).<ref name="ferre">{{cite journal | doi = 10.1159/000100426 | title = SREBP-1c Transcription Factor and Lipid Homeostasis: Clinical Perspective | journal = Hormone Research | year = 2007 | first = P. | last = Ferre |author2=F. Foufelle | volume = 68 | issue = 2 | pages = 72–82| doi-broken-date = 1 November 2024 | pmid = 17344645 | quote = this process is outlined graphically in page 73| doi-access = free }}</ref> This cytosolic acetyl-CoA can then be used to synthesize fatty acids through carboxylation by [[acetyl-CoA carboxylase]] into [[Malonyl-CoA|malonyl CoA]], the first committed step in the synthesis of fatty acids.<ref name="ferre" /><ref name="Voet">{{cite book |last=Voet |first=Donald |author2=Judith G. Voet |author3=Charlotte W. Pratt |title=Fundamentals of Biochemistry, 2nd Edition |publisher=John Wiley and Sons, Inc. |year=2006 |pages=[https://archive.org/details/fundamentalsofbi00voet_0/page/547 547, 556] |isbn=978-0-471-21495-3 |url=https://archive.org/details/fundamentalsofbi00voet_0/page/547 }}</ref> This conversion occurs primarily in the liver, [[adipose tissue]] and lactating [[mammary gland]]s, where the fatty acids are combined with [[glycerol]] to form [[triglyceride]]s, the major fuel reservoir of most animals. Fatty acids are also components of the [[phospholipid]]s that make up the bulk of the [[lipid bilayer]]s of all [[cellular membrane]]s.<ref name="stryer2" /> |
|||
** In plants, ''de novo'' fatty acid synthesis occurs in the [[plastid]]s. Many [[seed]]s accumulate large reservoirs of seed oils to support [[germination]] and early growth of the seedling before it is a net [[photosynthesis|photosynthetic]] organism. |
|||
** The [[cytosol]]ic acetyl-CoA can also condense with [[acetoacetyl-CoA]] to form 3-hydroxy-3-methylglutaryl-CoA ([[HMG-CoA]]) which is the rate-limiting step controlling the [[Mevalonate pathway|synthesis of cholesterol]].<ref name="stryer2" /> [[Cholesterol]] can be used as is, as a structural component of cellular membranes, or it can be used to synthesize [[Steroid#Steroidogenesis|steroid hormones]], [[Bile acids|bile salts]], and [[vitamin D]].<ref name="stryer2" /><ref name="Voet" /> |
|||
** Acetyl-CoA can be [[carboxylated]] in the cytosol by [[acetyl-CoA carboxylase]], giving rise to [[malonyl-CoA]], a substrate required for synthesis of [[flavonoid]]s and related [[polyketide]]s, for elongation of fatty acids to produce [[wax]]es, [[cuticle]], and seed oils in members of the [[Brassica]] family, and for [[malonate|malonation]] of proteins and other phytochemicals.<ref>{{cite journal|year=2005|title=Reverse Genetic Characterization of Cytosolic Acetyl-CoA Generation by ATP-Citrate Lyase in Arabidopsis|journal=The Plant Cell Online|volume=17|issue=1|pages=182–203|doi=10.1105/tpc.104.026211|pmid=15608338|last1=Fatland|first1=B. L.|pmc=544498}}</ref> In plants, these include [[sesquiterpene]]s, [[brassinosteroid]]s (hormones), and membrane [[sterol]]s. |
|||
* [[Steroid synthesis]]: |
|||
** Acetyl-CoA participates in the [[mevalonate pathway]] by partaking in the synthesis of hydroxymethyl glutaryl-CoA. |
|||
* [[Acetylcholine]] synthesis: |
|||
** Acetyl-CoA is also an important component in the biogenic synthesis of the [[neurotransmitter]] [[acetylcholine]]. [[Choline]], in combination with acetyl-CoA, is catalyzed by the enzyme [[choline acetyltransferase]] to produce acetylcholine and [[coenzyme A]] as a byproduct. |
|||
* [[Melatonin]] synthesis |
|||
* Acetylation |
|||
** Acetyl-CoA is also the source of the acetyl group incorporated onto certain [[lysine]] residues of [[histone]] and nonhistone proteins in the [[posttranslational modification]] [[acetylation]]. This acetylation is catalyzed by [[acetyltransferases]]. This acetylation affects [[cell growth]], [[mitosis]], and [[apoptosis]].<ref>{{cite journal|last1=Yi|first1=C. H.|last2=Vakifahmetoglu-Norberg|first2=H.|last3=Yuan|first3=J.|date=2011-01-01|title=Integration of Apoptosis and Metabolism|journal=Cold Spring Harbor Symposia on Quantitative Biology|language=en|volume=76|pages=375–387|doi=10.1101/sqb.2011.76.010777|issn=0091-7451|pmid=22089928|doi-access=free}}</ref> |
|||
*Allosteric regulator |
|||
** Acetyl-CoA serves as an [[allosteric regulation|allosteric regulator]] of [[pyruvate dehydrogenase kinase]] (PDK). It regulates through the ratio of acetyl-CoA versus CoA. Increased concentration of acetyl-CoA activates PDK.<ref>{{cite journal|last1=Pettit|first1=Flora H.|last2=Pelley|first2=John W.|last3=Reed|first3=Lester J.|date=1975-07-22|title=Regulation of pyruvate dehydrogenase kinase and phosphatase by acetyl-CoA/CoA and NADH/NAD ratios|journal=Biochemical and Biophysical Research Communications|volume=65|issue=2|pages=575–582|doi=10.1016/S0006-291X(75)80185-9|pmid=167775}}</ref> |
|||
** Acetyl-CoA is also an allosteric activator of [[pyruvate carboxylase]].<ref>{{cite journal|last1=Jitrapakdee|first1=Sarawut|last2=Maurice|first2=Martin St.|author3-link=Ivan Rayment|last3=Rayment|first3=Ivan|last4=Cleland|first4=W. Wallace|last5=Wallace|first5=John C.|last6=Attwood|first6=Paul V.|date=2008-08-01|title=Structure, Mechanism and Regulation of Pyruvate Carboxylase|journal=The Biochemical Journal|volume=413|issue=3|pages=369–387|doi=10.1042/BJ20080709|issn=0264-6021|pmc=2859305|pmid=18613815}}</ref> |
|||
==Interactive pathway map== |
|||
''Click on genes, proteins and metabolites below to visit [[Portal:Gene Wiki|Gene Wiki]] pages and related Wikipedia articles. The pathway can be downloaded and edited at [http://www.wikipathways.org WikiPathways].'' |
|||
{| style="margin-left: auto; margin-right: auto; border: none;" |
|||
| width="390px"|{{TCACycle_WP78|highlight=Acetyl-CoA|header=}} |
|||
| width="390px"|{{StatinPathway_WP430|highlight=Acetyl-coa|header=}} |
|||
|} |
|||
==See also== |
|||
* [[Malonyl-CoA decarboxylase]] |
* [[Malonyl-CoA decarboxylase]] |
||
| Line 83: | Line 128: | ||
* {{MeshName|Acetyl+Coenzyme+A}} |
* {{MeshName|Acetyl+Coenzyme+A}} |
||
{{Fatty-acid metabolism intermediates}} |
|||
{{Nootropics}} |
|||
{{Cholesterol metabolism intermediates}} |
{{Cholesterol metabolism intermediates}} |
||
{{Glycolysis}} |
{{Glycolysis}} |
||
{{Citric acid cycle}} |
|||
{{Amino acid metabolism intermediates}} |
{{Amino acid metabolism intermediates}} |
||
{{Acetylcholine receptor modulators}} |
|||
{{Cholinergics}} |
|||
{{Authority control}} |
|||
{{DEFAULTSORT:Acetyl-Coa}} |
{{DEFAULTSORT:Acetyl-Coa}} |
||
[[Category:Cholinergics]] |
|||
[[Category:Metabolism]] |
[[Category:Metabolism]] |
||
[[Category:Thioesters]] |
[[Category:Thioesters of coenzyme A]] |
||
[[Category: |
[[Category:Glycolysis]] |
||
[[Category:Metabolic intermediates]] |
|||
[[Category:Acetyl compounds]] |
|||
[[ca:Acetil-CoA]] |
|||
[[cs:Acetylkoenzym A]] |
|||
[[de:Coenzym A#Acetyl-CoA]] |
|||
[[et:Atsetüülkoensüüm A]] |
|||
[[es:Acetil-CoA]] |
|||
[[eo:Acetila A-koenzimo]] |
|||
[[eu:Azetil-CoA]] |
|||
[[fr:Acétyl-coenzyme A]] |
|||
[[gl:Acetil-CoA]] |
|||
[[ko:아세틸 조효소 A]] |
|||
[[id:Koenzim-A asetil]] |
|||
[[it:Acetil-coenzima A]] |
|||
[[he:אצטיל קואנזים A]] |
|||
[[lb:HSCoA]] |
|||
[[hu:Acetil-koenzim A]] |
|||
[[nl:Acetyl-CoA]] |
|||
[[ja:アセチルCoA]] |
|||
[[pt:Acetilcoenzima A]] |
|||
[[fi:Asetyylikoentsyymi-A]] |
|||
[[sv:Acetyl-CoA]] |
|||
[[tr:Asetil-KoA]] |
|||
[[zh:乙酰辅酶A]] |
|||
Latest revision as of 03:05, 2 November 2024
This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages)
|

| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
O1-{(3R)-4-[(3-{[2-(Acetylsulfanyl)ethyl]amino}-3-oxopropyl)amino]-3-hydroxy-2,2-dimethyl-4-oxobutyl} O3-{[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methyl} dihydrogen diphosphate | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.000.719 |
| KEGG | |
| MeSH | Acetyl+Coenzyme+A |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C23H38N7O17P3S | |
| Molar mass | 809.57 g·mol−1 |
| UV-vis (λmax) | 260 nm; 232 nm[1] |
| Absorbance | ε260 = 16.4 mM−1 cm−1 (adenosine)[1] ε232 = 8.7 mM−1 cm−1 (thioester)[1] Δε232 on thioester hydrolysis = −4.5 mM−1 cm−1[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism.[2] Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized for energy production.
Coenzyme A (CoASH or CoA) consists of a β-mercaptoethylamine group linked to pantothenic acid (vitamin B5) through an amide linkage[3] and 3'-phosphorylated ADP. The acetyl group (indicated in blue in the structural diagram on the right) of acetyl-CoA is linked to the sulfhydryl substituent of the β-mercaptoethylamine group. This thioester linkage is a "high energy" bond, which is particularly reactive. Hydrolysis of the thioester bond is exergonic (−31.5 kJ/mol).
CoA is acetylated to acetyl-CoA by the breakdown of carbohydrates through glycolysis and by the breakdown of fatty acids through β-oxidation. Acetyl-CoA then enters the citric acid cycle, where the acetyl group is oxidized to carbon dioxide and water, and the energy released is captured in the form of 11 ATP and one GTP per acetyl group.
Konrad Bloch and Feodor Lynen were awarded the 1964 Nobel Prize in Physiology or Medicine for their discoveries linking acetyl-CoA and fatty acid metabolism. Fritz Lipmann won the Nobel Prize in 1953 for his discovery of the cofactor coenzyme A.[4]
Role
[edit]Acetyl-CoA is a metabolic intermediate that is involved in many metabolic pathways in an organism. It is produced during the breakdown of glucose, fatty acids, and amino acids, and is used in the synthesis of many other biomolecules, including cholesterol, fatty acids, and ketone bodies. Acetyl-CoA is also a key molecule in the citric acid cycle, which is a series of chemical reactions that occur in the mitochondria of cells and is responsible for generating energy in the form of ATP.[5][6]
In addition, acetyl-CoA is a precursor for the biosynthesis of various acetyl-chemicals, acting as an intermediate to transfer an acetyl group during the biosynthesis of those acetyl-chemicals. Acetyl-CoA is also involved in the regulation of various cellular mechanisms by providing acetyl groups to target amino acid residues for post-translational acetylation reactions of proteins.
Biosynthesis
[edit]The acetylation of CoA is determined by the carbon sources.[7][8]
Extramitochondrial
[edit]- At high glucose levels, glycolysis takes place rapidly, thus increasing the amount of citrate produced from the citric acid cycle. This citrate is then exported to other organelles outside the mitochondria to be broken into acetyl-CoA and oxaloacetate by the enzyme ATP citrate lyase (ACL). This principal reaction is coupled with the hydrolysis of ATP.[9][10]
- At low glucose levels:
- CoA is acetylated using acetate by acetyl-CoA synthetase (ACS), also coupled with ATP hydrolysis.[11]
- Ethanol also serves as a carbon source for acetylation of CoA utilizing the enzyme alcohol dehydrogenase.[12]
- Degradation of branched-chain ketogenic amino acids such as valine, leucine, and isoleucine occurs. These amino acids are converted to α-ketoacids by transamination and eventually to isovaleryl-CoA through oxidative decarboxylation by an α-ketoacid dehydrogenase complex. Isovaleryl-CoA undergoes dehydrogenation, carboxylation and hydration to form another CoA-derivative intermediate before it is cleaved into acetyl-CoA and acetoacetate.[13][page needed]
Intramitochondrial
[edit]
- At high glucose levels, acetyl-CoA is produced through glycolysis.[14] Pyruvate undergoes oxidative decarboxylation in which it loses its carboxyl group (as carbon dioxide) to form acetyl-CoA, giving off 33.5 kJ/mol of energy. The oxidative conversion of pyruvate into acetyl-CoA is referred to as the pyruvate dehydrogenase reaction. It is catalyzed by the pyruvate dehydrogenase complex. Other conversions between pyruvate and acetyl-CoA are possible. For example, pyruvate formate lyase disproportionates pyruvate into acetyl-CoA and formic acid.

- At low glucose levels, the production of acetyl-CoA is linked to β-oxidation of fatty acids. Fatty acids are first converted to acyl-CoA. Acyl-CoA is then degraded in a four-step cycle of oxidation, hydration, oxidation and thiolysis catalyzed by four respective enzymes, namely acyl-CoA dehydrogenase, enoyl-CoA hydratase, 3-hydroxyacyl-CoA dehydrogenase, and thiolase. The cycle produces a new fatty acid chain with two fewer carbons and acetyl-CoA as a byproduct.[15]
Functions
[edit]Intermediates in various pathways
[edit]- In cellular respiration
- Citric acid cycle:
- Through a series of chemical reactions, stored energy is released through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins into adenosine triphosphate (ATP) and carbon dioxide.
- Fatty acid metabolism
- Acetyl-CoA is produced by the breakdown of both carbohydrates (by glycolysis) and lipids (by β-oxidation). It then enters the citric acid cycle in the mitochondrion by combining with oxaloacetate to form citrate.[16][17]
- Two acetyl-CoA molecules condense to form acetoacetyl-CoA, which gives rise to the formation of acetoacetate and β-hydroxybutyrate.[16] Acetoacetate, β-hydroxybutyrate, and their spontaneous breakdown product acetone[18] are frequently, but confusingly, known as ketone bodies (as they are not "bodies" at all, but water-soluble chemical substances). The ketone bodies are released by the liver into the blood. All cells with mitochondria can take ketone bodies up from the blood and reconvert them into acetyl-CoA, which can then be used as fuel in their citric acid cycles, as no other tissue can divert its oxaloacetate into the gluconeogenic pathway in the way that the liver does. Unlike free fatty acids, ketone bodies can cross the blood–brain barrier and are therefore available as fuel for the cells of the central nervous system, acting as a substitute for glucose, on which these cells normally survive.[16] The occurrence of high levels of ketone bodies in the blood during starvation, a low-carbohydrate diet, prolonged heavy exercise, and uncontrolled type-1 diabetes mellitus is known as ketosis, and in its extreme form in out-of-control type-1 diabetes mellitus, as ketoacidosis.
- On the other hand, when the insulin concentration in the blood is high, and that of glucagon is low (i.e. after meals), the acetyl-CoA produced by glycolysis condenses as normal with oxaloacetate to form citrate in the mitochondrion. However, instead of continuing through the citric acid cycle to be converted to carbon dioxide and water, the citrate is removed from the mitochondrion into the cytoplasm.[16] There it is cleaved by ATP citrate lyase into acetyl-CoA and oxaloacetate. The oxaloacetate is returned to the mitochondrion as malate (and then converted back into oxaloacetate to transfer more acetyl-CoA out of the mitochondrion).[19] This cytosolic acetyl-CoA can then be used to synthesize fatty acids through carboxylation by acetyl-CoA carboxylase into malonyl CoA, the first committed step in the synthesis of fatty acids.[19][20] This conversion occurs primarily in the liver, adipose tissue and lactating mammary glands, where the fatty acids are combined with glycerol to form triglycerides, the major fuel reservoir of most animals. Fatty acids are also components of the phospholipids that make up the bulk of the lipid bilayers of all cellular membranes.[16]
- In plants, de novo fatty acid synthesis occurs in the plastids. Many seeds accumulate large reservoirs of seed oils to support germination and early growth of the seedling before it is a net photosynthetic organism.
- The cytosolic acetyl-CoA can also condense with acetoacetyl-CoA to form 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) which is the rate-limiting step controlling the synthesis of cholesterol.[16] Cholesterol can be used as is, as a structural component of cellular membranes, or it can be used to synthesize steroid hormones, bile salts, and vitamin D.[16][20]
- Acetyl-CoA can be carboxylated in the cytosol by acetyl-CoA carboxylase, giving rise to malonyl-CoA, a substrate required for synthesis of flavonoids and related polyketides, for elongation of fatty acids to produce waxes, cuticle, and seed oils in members of the Brassica family, and for malonation of proteins and other phytochemicals.[21] In plants, these include sesquiterpenes, brassinosteroids (hormones), and membrane sterols.
- Steroid synthesis:
- Acetyl-CoA participates in the mevalonate pathway by partaking in the synthesis of hydroxymethyl glutaryl-CoA.
- Acetylcholine synthesis:
- Acetyl-CoA is also an important component in the biogenic synthesis of the neurotransmitter acetylcholine. Choline, in combination with acetyl-CoA, is catalyzed by the enzyme choline acetyltransferase to produce acetylcholine and coenzyme A as a byproduct.
- Melatonin synthesis
- Acetylation
- Acetyl-CoA is also the source of the acetyl group incorporated onto certain lysine residues of histone and nonhistone proteins in the posttranslational modification acetylation. This acetylation is catalyzed by acetyltransferases. This acetylation affects cell growth, mitosis, and apoptosis.[22]
- Allosteric regulator
- Acetyl-CoA serves as an allosteric regulator of pyruvate dehydrogenase kinase (PDK). It regulates through the ratio of acetyl-CoA versus CoA. Increased concentration of acetyl-CoA activates PDK.[23]
- Acetyl-CoA is also an allosteric activator of pyruvate carboxylase.[24]
Interactive pathway map
[edit]Click on genes, proteins and metabolites below to visit Gene Wiki pages and related Wikipedia articles. The pathway can be downloaded and edited at WikiPathways.
|
TCACycle_WP78 edit
|
Statin pathway edit
|
See also
[edit]References
[edit]- ^ a b c d Dawson RM, Elliott DC, Elliott WH, Jones KM (2002). Data for Biochemical Research (3rd ed.). Clarendon Press. p. 117. ISBN 978-0-19-855299-4.
- ^ "Acetyl CoA Crossroads". chemistry.elmhurst.edu. Archived from the original on 2016-11-15. Retrieved 2016-11-08.
- ^ "Fatty Acids -- Structure of Acetyl CoA". library.med.utah.edu. Retrieved 2017-06-02.
- ^ "All Nobel Prizes in Physiology or Medicine". The Nobel Prize.
- ^ Zhang S, Yang W, Chen H, Liu B, Lin B, Tao Y (August 2019). "Metabolic engineering for efficient supply of acetyl-CoA from different carbon sources in Escherichia coli". Microb Cell Fact. 18 (1): 130. doi:10.1186/s12934-019-1177-y. PMC 6685171. PMID 31387584.
- ^ "5.12G: The Acetyl-CoA Pathway". 9 May 2017.
- ^ Hynes MJ, Murray SL (2010-07-01). "ATP-Citrate Lyase Is Required for Production of Cytosolic Acetyl Coenzyme A and Development in Aspergillus nidulans". Eukaryotic Cell. 9 (7): 1039–1048. doi:10.1128/EC.00080-10. ISSN 1535-9778. PMC 2901662. PMID 20495057.
- ^ Wellen KE, Thompson CB (2012-04-01). "A two-way street: reciprocal regulation of metabolism and signalling". Nature Reviews Molecular Cell Biology. 13 (4): 270–276. doi:10.1038/nrm3305. ISSN 1471-0072. PMID 22395772. S2CID 244613.
- ^ Storey KB (2005-02-25). Functional Metabolism: Regulation and Adaptation. John Wiley & Sons. ISBN 9780471675570.
- ^ "ACLY ATP citrate lyase [Homo sapiens (human)] - Gene - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2016-11-06.
- ^ Ragsdale SW (2004). "Life with carbon monoxide". CRC Critical Reviews in Biochemistry and Molecular Biology. 39 (3): 165–195. doi:10.1080/10409230490496577. PMID 15596550. S2CID 16194968.
- ^ Chatterjea (2004-01-01). Textbook of Biochemistry for Dental/Nursing/Pharmacy Students. Jaypee Brothers Publishers. ISBN 9788180612046.[permanent dead link]
- ^ Berg JM, Tymoczko JL, Stryer L (2002). Biochemistry (5th ed.). W. H. Freeman. ISBN 978-0716730514.
- ^ Blackstock JC (2014-06-28). Guide to Biochemistry. Butterworth-Heinemann. ISBN 9781483183671.
- ^ Houten SM, Wanders RJ (2010-03-02). "A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation". Journal of Inherited Metabolic Disease. 33 (5): 469–477. doi:10.1007/s10545-010-9061-2. ISSN 0141-8955. PMC 2950079. PMID 20195903.
- ^ a b c d e f g Stryer L (1995). Biochemistry (Fourth ed.). New York: W.H. Freeman and Company. pp. 510–515, 559–565, 581–613, 614–623, 775–778. ISBN 978-0-7167-2009-6.
- ^ "Oxidation of fatty acids". 2013-10-11.
- ^ "Ketone body metabolism". University of Waterloo.
- ^ a b Ferre P, F. Foufelle (2007). "SREBP-1c Transcription Factor and Lipid Homeostasis: Clinical Perspective". Hormone Research. 68 (2): 72–82. doi:10.1159/000100426 (inactive 1 November 2024). PMID 17344645.
this process is outlined graphically in page 73
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ a b Voet D, Judith G. Voet, Charlotte W. Pratt (2006). Fundamentals of Biochemistry, 2nd Edition. John Wiley and Sons, Inc. pp. 547, 556. ISBN 978-0-471-21495-3.
- ^ Fatland BL (2005). "Reverse Genetic Characterization of Cytosolic Acetyl-CoA Generation by ATP-Citrate Lyase in Arabidopsis". The Plant Cell Online. 17 (1): 182–203. doi:10.1105/tpc.104.026211. PMC 544498. PMID 15608338.
- ^ Yi CH, Vakifahmetoglu-Norberg H, Yuan J (2011-01-01). "Integration of Apoptosis and Metabolism". Cold Spring Harbor Symposia on Quantitative Biology. 76: 375–387. doi:10.1101/sqb.2011.76.010777. ISSN 0091-7451. PMID 22089928.
- ^ Pettit FH, Pelley JW, Reed LJ (1975-07-22). "Regulation of pyruvate dehydrogenase kinase and phosphatase by acetyl-CoA/CoA and NADH/NAD ratios". Biochemical and Biophysical Research Communications. 65 (2): 575–582. doi:10.1016/S0006-291X(75)80185-9. PMID 167775.
- ^ Jitrapakdee S, Maurice MS, Rayment I, Cleland WW, Wallace JC, Attwood PV (2008-08-01). "Structure, Mechanism and Regulation of Pyruvate Carboxylase". The Biochemical Journal. 413 (3): 369–387. doi:10.1042/BJ20080709. ISSN 0264-6021. PMC 2859305. PMID 18613815.
External links
[edit]- Acetyl+Coenzyme+A at the U.S. National Library of Medicine Medical Subject Headings (MeSH)