Indoxacarb: Difference between revisions
m Chembox: move all ATCCode-parameters into section Parmacology. See WP:Chembox talk#Drugbank_and_ATC_positioning (+minor param corrections) (via AWB script) |
Cyberbot II (talk | contribs) Rescuing 1 sources, flagging 0 as dead, and archiving 1 sources. #IABot |
||
| Line 97: | Line 97: | ||
}} |
}} |
||
'''Indoxacarb''' is an oxadiazine [[pesticide]] developed by [[DuPont]] that acts against [[lepidoptera]]n larvae. It is marketed under the names '''Indoxacarb Technical''' Insecticide, '''Steward''' Insecticide and '''Avaunt''' Insecticide. It is also used as the active ingredient in Syngenta line of commercial pesticides: '''Advion''' and '''Arilon'''.<ref>[http://www.epa.gov/opprd001/factsheets/indoxacarb.pdf United States Environmental Protection Agency. Office of Prevention, Pesticides and Toxic Substances (7505C). Pesticide Fact Sheet. Name of Chemical: Indoxacarb. Reason for Issuance: Conditional Registration. Date Issued: October 30, 2000. ]{{ |
'''Indoxacarb''' is an oxadiazine [[pesticide]] developed by [[DuPont]] that acts against [[lepidoptera]]n larvae. It is marketed under the names '''Indoxacarb Technical''' Insecticide, '''Steward''' Insecticide and '''Avaunt''' Insecticide. It is also used as the active ingredient in Syngenta line of commercial pesticides: '''Advion''' and '''Arilon'''.<ref>[http://www.epa.gov/opprd001/factsheets/indoxacarb.pdf United States Environmental Protection Agency. Office of Prevention, Pesticides and Toxic Substances (7505C). Pesticide Fact Sheet. Name of Chemical: Indoxacarb. Reason for Issuance: Conditional Registration. Date Issued: October 30, 2000. ] {{wayback|url=http://www.epa.gov/opprd001/factsheets/indoxacarb.pdf |date=20040502142848 }}</ref><ref>[http://www.epa.gov/EPA-PEST/2007/July/Day-11/p13339.htm United States Environmental Protection Agency. Federal Register: Indoxacarb; Pesticide Tolerance. Federal Register: July 11, 2007 (Volume 72, Number 132) ]{{Dead link|date=October 2015}}</ref><ref>[http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:025:0024:0027:EN:PDF Commission Directive 2006/10/EC of 27 January 2006 amending Council Directive 91/414/EEC to include forchlorfenuron and indoxacarb as active substances. Official Journal of the European Union 2006-1-28]</ref> |
||
Its main mode of action is via blocking of nerve [[sodium channel]]s. It is fairly [[lipophilic]] with a [[dissociation constant|K<sub>ow</sub>]] of 4.65. |
Its main mode of action is via blocking of nerve [[sodium channel]]s. It is fairly [[lipophilic]] with a [[dissociation constant|K<sub>ow</sub>]] of 4.65. |
||
Revision as of 01:26, 22 January 2016
| |
| Names | |
|---|---|
| Preferred IUPAC name
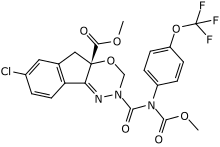
Methyl 7-chloro-2,5-dihydro-2-[[(methoxycarbonyl)[4-(trifluoromethoxy)phenyl]amino]carbonyl]indeno[1,2-e][1,3,4]oxadiazine-4a(3H)-carboxylate | |
| Systematic IUPAC name
(S)-Methyl 7-chloro-2-{[(methoxycarbonyl)[4-(trifluoromethoxy)phenyl]amino]carbonyl}-2H,3H,4aH,5H-indeno[1,2-e][1,3,4]oxadiazine-4a-carboxylate | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | DPX-MP062 |
| 8366683 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.132.370 |
| KEGG | |
| MeSH | Indoxacarb |
PubChem CID
|
|
| UNII | |
| UN number | UN 3077 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C22H17ClF3N3O7 | |
| Molar mass | 527.84 g·mol−1 |
| Melting point | 88.1 °C (190.6 °F; 361.2 K) 99% indoxacarb PAI |
| Pharmacology | |
| QP53AX27 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Indoxacarb is an oxadiazine pesticide developed by DuPont that acts against lepidopteran larvae. It is marketed under the names Indoxacarb Technical Insecticide, Steward Insecticide and Avaunt Insecticide. It is also used as the active ingredient in Syngenta line of commercial pesticides: Advion and Arilon.[1][2][3]
Its main mode of action is via blocking of nerve sodium channels. It is fairly lipophilic with a Kow of 4.65.
Household products
Indoxacarb is the active ingredient in a number of household insecticides, including cockroach and ant baits, and can remain active after digestion. [4] In 2012 DuPont's Professional Products including the line of Advion and Arilon products was purchased by Syngenta.[5] Indoxacarb is the active ingredient in the new pet product, Activyl from Merck Animal Health. It is marketed to kill fleas on dogs and cats.[6]
References
- ^ United States Environmental Protection Agency. Office of Prevention, Pesticides and Toxic Substances (7505C). Pesticide Fact Sheet. Name of Chemical: Indoxacarb. Reason for Issuance: Conditional Registration. Date Issued: October 30, 2000. Archived 2004-05-02 at the Wayback Machine
- ^ United States Environmental Protection Agency. Federal Register: Indoxacarb; Pesticide Tolerance. Federal Register: July 11, 2007 (Volume 72, Number 132) [dead link]
- ^ Commission Directive 2006/10/EC of 27 January 2006 amending Council Directive 91/414/EEC to include forchlorfenuron and indoxacarb as active substances. Official Journal of the European Union 2006-1-28
- ^ "Indoxacarb Insecticide Wipes Out Entire Cockroach Generations". June 23, 2008. Retrieved 2009-12-14.
- ^ http://www3.syngenta.com/country/au/en/news/releases/Pages/Syngenta-acquires-DuPont-Professional-Products.aspx
- ^ http://us.activyl.com
Further reading
- Lapied, Bruno; Françoise Grolleau; David B Sattelle (January 2001). "Indoxacarb, an oxadiazine insecticide, blocks insect neuronal sodium channels". Br J Pharmacol. 132 (2): 587–595. doi:10.1038/sj.bjp.0703853. PMC 1572588. PMID 11159709.
{{cite journal}}: Cite has empty unknown parameters:|laysource=,|laydate=,|laysummary=, and|quotes=(help) - Khambay, Bhupinder P.S. (2002). "Pyrethroid Insecticides". Pesticide Outlook. 13 (2): 49–54. doi:10.1039/b202996k.
{{cite journal}}: Cite has empty unknown parameters:|laysource=,|laydate=,|laysummary=, and|quotes=(help) - Moncada, Adriana. Environmental Fate of Indoxacarb. Environmental Monitoring Branch, Department of Pesticide Regulation, State of California. March 6, 2003
- Tillman, P Glynn (January 2002). "Toxicity of a formulation of the insecticide indoxacarb to the tarnished plant bug, Lygus lineolaris (Hemiptera: Miridae), and the big-eyed bug, Geocoris punctipes (Hemiptera: Lygaeidae)". Pest-Manag-Sci. 58 (1): 92–100. doi:10.1002/ps.426. PMID 11838290.
{{cite journal}}: Cite has empty unknown parameters:|laysource=,|laydate=,|laysummary=, and|quotes=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help)
External links
- DuPont Steward insecticide - FAQs. Updated 20 January 2007. Retrieved 2012-11-11
- Indoxacarb in the Pesticide Properties DataBase (PPDB)
