TAAR1
| TAAR1 | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||
| Identifikatori | |||||||||||||||||||||||||
| Aliasi | TAAR1 | ||||||||||||||||||||||||
| Vanjski ID-jevi | OMIM: 609333 MGI: 2148258 HomoloGene: 24938 GeneCards: TAAR1 | ||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Ortolozi | |||||||||||||||||||||||||
| Vrste | Čovjek | Miš | |||||||||||||||||||||||
| Entrez | |||||||||||||||||||||||||
| Ensembl | |||||||||||||||||||||||||
| UniProt | |||||||||||||||||||||||||
| RefSeq (mRNK) | |||||||||||||||||||||||||
| RefSeq (bjelančevina) | |||||||||||||||||||||||||
| Lokacija (UCSC) | Chr 6: 132.64 – 132.66 Mb | Chr 10: 23.8 – 23.8 Mb | |||||||||||||||||||||||
| PubMed pretraga | [3] | [4] | |||||||||||||||||||||||
| Wikipodaci | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
Receptor 1 pridružen tragovima amina (TAAR1) jest protein koji je kod ljudi kodiran genom TAAR1.[5] TAAR1 je unutarćelijski aminski aktivirana Gs-pridruženi i Gq pridruženi G-protein spregnuti receptor (GPCR) koji se primarno eksprimira u nekoliko perifernih organa i ćelija (npr. želudac, tanko crijevo , duodenum i bijela krvna zrnca), astrocitima i u unutarćelijskom okruženju unutar presinapsne plazmamembrane (tj. aksonski terminal) monoaminski neuroni u centralnom nervnom sistemu (CNS).[6][7][8] TAAR1 su 2001. otkrile dvije nezavisne grupe istraživača, Borowski et al. i Bunzow et al.[9][10] TAAR1 je jedna od šest funkcionalnih receptora povezanih s aminima u tragovima, koji su tako nazvani po svojoj sposobnosti vezivanja endogenih amina, koji se javljaju u tkivima u koncentracijama u tragovima.[11][12] TAAR1 ima značajnu ulogu u regulaciji neurotransmisije u dopaminskim, norepinefrinskim i serotoninskim neuronima u CNS-u;[11][13] također utiče na imunski sistem i neuroimunski sistem putem različitih mehanizama.[14][15][16][17]
TAAR1 je receptor visokog afiniteta za amfetamin, metamfetamin, dopamin i u tragovima amin, koji posreduje u nekim njihovim ćelijskim efektima u monoaminskim neuronima unutar centralnog nervnog sistema.[11][13]
Primarni endogeni ligandi ljudskog receptora za TAAR1 (hTAAR1), prema redoslijedu jačine, su tiramin> β-fenetilamin >dopamin i oktopamin.[6]
Otkriće
[uredi | uredi izvor]TAAR1 su nezavisno otkrili Borowski et al. i Bunzow et al., 2001. Da bi pronašli genetičke varijante odgovorne za sintezu TAAR1, koristili su mješavine oligonukleotida sa sekvencama vezanim za G-protein spregnuti receptor (GPCR) serotonina i dopamina za otkrivanje novih sekvenci DNK u pacovske genomske DNK i cDNK, koju su zatim pojačali i klonirali. Rezultirajuća sekvenca nije pronađena u bilo kojoj bazi podataka i kodiran je kao TAAR1.[9][10] Kasnije je funkcionalnu ulogu TAAR1 i drugih receptora iz ove porodice proučavali su Raul Gainetdinov i njegove kolege.[18]
Aminokiselinska sekvenca
[uredi | uredi izvor]Dužina polipeptidnog lanca je 339 aminokiselina, а molekulska težina 39.092 Da.[19]
| 10 | 20 | 30 | 40 | 50 | ||||
|---|---|---|---|---|---|---|---|---|
| MMPFCHNIIN | ISCVKNNWSN | DVRASLYSLM | VLIILTTLVG | NLIVIVSISH | ||||
| FKQLHTPTNW | LIHSMATVDF | LLGCLVMPYS | MVRSAEHCWY | FGEVFCKIHT | ||||
| STDIMLSSAS | IFHLSFISID | RYYAVCDPLR | YKAKMNILVI | CVMIFISWSV | ||||
| PAVFAFGMIF | LELNFKGAEE | IYYKHVHCRG | GCSVFFSKIS | GVLTFMTSFY | ||||
| IPGSIMLCVY | YRIYLIAKEQ | ARLISDANQK | LQIGLEMKNG | ISQSKERKAV | ||||
| KTLGIVMGVF | LICWCPFFIC | TVMDPFLHYI | IPPTLNDVLI | WFGYLNSTFN | ||||
| PMVYAFFYPW | FRKALKMMLF | GKIFQKDSSR | CKLFLELSS |
Struktura
[uredi | uredi izvor]TAAR1 ima strukturne sličnosti sa receptorima sličnim rodopsinu, klasa A rodopsina potporodica GPCR.<ef name="Bunzow_2001"/> Ima sedam transmembranska domena sa kratkim N– i C-terminalnim proširenjima.[20] TAAR1 je 62–96% identičan s TAARs2-15, što sugerira da je TAAR potporodica nedavno evoluirala; dok u isto vrijeme, nizak stupanj sličnosti između TAAR1 ortolozi sugeriraju da brzo evoluiraju.[9] TAAR1 dijeli prediktivni peptid motiv sa svim ostalim TAAR-ovima. Ovaj se motiv preklapa s transmembranskom domenom VII, a njegov identitet je NSXXNPXX [Y, H] XXX [Y, F] XWF. TAAR1 i njegovi homolozi imaju ligandne džepne vektore koji koriste skupove od 35 aminokiselina za koje je poznato da su direktno uključene u interakciju receptor-ligand.[12]
Gen
[uredi | uredi izvor]Svi ljudski TAAR geni nalaze se na jednom hromosomu koji obuhvata 109 kb ljudskog hromosoma 6 regija 6q23.1, od 192 kb i mišjeg hromosoma 10A4 i 216 kb pacovskog hromosoma 1p12. Svaki TAAR je izveden iz jednog egzona, osim TAAR2, koji je kodiran s dva egzona.[12] Smatra se da je ljudski gen "TAAR1" ima intron manje.[21]
Tkivna distribucija
[uredi | uredi izvor]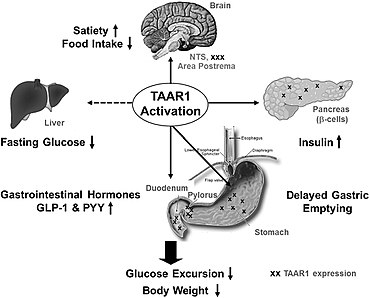
Do danas je TAAR1 identifikovan i kloniran u pet različitih sisarskih genoma: ljudi, miševi, pacovi, majmuni i čimpanze. Kod pacova se iRNK za TAAR1 nalazi u niskim do umjerenim nivoima u perifernim tkivima, kao što su želudac, bubreg, crijeva[22] i pluća i na niskim nivoima u mozgu.[9] rezus-makakov Taar1 i ljudski TAAR1 imaju veliku sličnost sekvence, a TAAR1 iRNK je visoko eksprimirana u iste važne monoaminergijske regije obje vrste. Ove regije uključuju dorzalnu i ventralnu stranu dijelova kao što su kaudalno jezgro, putamen, substantia nigra, nucleus accumbens, ventralna tegmentna površina, locus coeruleus, amigdala i raphe nucleus.[6][23] hTAAR1 je također identificiran u ljudskim astrocitima.[6][14] Izvan ljudskog centralnog nervnog sistema, hTAAR1 se također javlja kao unutarćelijski receptor i primarno se eksprimira u želucu, crijevima,[22] duodenumu,< ref name="Bugda Gwilt_2019"/> pankreasnim β-ćelijama i bijelim krvnim zrncima.[8][22] U duodenumu, aktivacija TAAR1 povećava peptidu sličan glukagonu-1 (GLP-1) i peptid YY (PYY) editiranje;[8] u želucu je primijećeno da aktivacija hTAAR1 povećava somatostatin (rast hormon koji inhibira hormon)) iz delta ćelija.[8]
hTAAR1 je jedini podtip receptora povezanog s ljuskim aminima koji nije eksprimiran unutar ljudskog mirisnog epitela.[24]
Lokacija unutar neurona
[uredi | uredi izvor]TAAR1 je unutarćelijski receptor unutar presinapsnog terminala monoaminskih neurona kod ljudi i drugih životinja.[11][13][25] U modelu ćelijskih sistema, hTAAR1 ima izrazito lošu membransku ekspresiju.[25] Metod induciranja membranske ekspresije hTAAR1korišten je za proučavanje njene farmakologije pomoću transmisije energijske rezonancije cAMP-a.[25]
Budući da je TAAR1 unutarćelijski receptor u monoaminskim neuronima, egzogeni ljudski TAAR1 ligandi moraju ući u presinapsni dio neuron kroz membranski transportni protein. U dopaminskim, norepinefrinskim i serotoninskim neuronima, primarni membranski transporteri su DAT, NET i SERT.[11] | group = "note"}} ili moći difundirati po presinapsnoj membrani kako bi dosegao receptor i proizveo inhibiciju ponovnog preuzimanja i isticanje neurotransmitera.[11] Shodno tome, učinkovitost određenog TAAR1 liganda u stvaranju ovih efekata na različitim monoaminskim neuronima funkcija je i njegovog afiniteta vezanja na TAAR1 i njegove sposobnosti kretanja po presinapsnoj membrani na svakom tipu neurona.[11] Varijabilnost između afiniteta supstrata TAAR1 liganda prema različitim transporterima monoamina je uveliko različita u njegovoj sposobnosti da proizvede oslobađanje i inhibiciju ponovnog preuzimanja neurotransmitera u različitim tipovima monoaminskih neurona.[11] Npr., TAAR1 ligand koji lahko može proći kroz transporter norepinefrina, ali ne i kroz serotoninski transporter, proizvest će - sve ostale jednake – izrazito veće efekte izazvane TAAR1 u noradrenalinskim neuronima u odnosu na neurone serotonina.
Receptorski oligomeri
[uredi | uredi izvor]hTAAR1 je jedini podtip receptora povezanih s ljudskim aminima koji nije izrađen unutar ljudskog mirisnog epitela.[24]
Ligandi
[uredi | uredi izvor]| Receptor 1 pridružen aminu u tragu | |
|---|---|
| Identifikatori | |
| Simbol | TAAR1
|
Agonisti
[uredi | uredi izvor]Amini u tragovima
[uredi | uredi izvor]Amini u tragovima su endogeni amini koji djeluju kao agonisti na TAAR1 i prisutni su u vanćelijskih koncentracija od 0,1–10 nanomolara u mozgu, što je manje od 1% ukupnih biogenih aminskih tvari u sisarskom nervnom sistemu.[26] Neki od ljudskih amina u tragovima uključuju triptamin, fenetilamin (PEA), N-metilfenetilamin, p-tiramin, m-tiramin, N-metiltiramin, p-oktopamin, m-oktopamin, i sinefrin. Oni imaju strukturne sličnosti sa tri uobičajena monoamina: serotonin, dopamin i norepinefrin. Svaki ligand ima različitu moć, mjerenu kao povećana koncentracija cikličnog AMP-a (cAMP) nakon vezivanja.
Redoslijed potencijala primarnih endogenih liganda u ljudskom TAAR1 je: tiramin > β-fenetilamin > dopamin > oktopamin.[6]
Tironamini
[uredi | uredi izvor]Tironamini su molekulni derivati hormona štitnjače i vrlo su važni za funkciju endokrinog sistema. 3-Jodotyronamin (T1AM) je najmoćniji agonist TAAR1 do sada otkriven, iako mu nedostaje afinitet prema monoaminskim transporterima i stoga ima mali učinak na monoaminske neurone centralnog nervnog sistema. Aktiviranje TAAR1 putem T1AM rezultira proizvodnjom velikih količina cAMP-a. Ovaj učinak povezan je sa smanjenjem tjelesne temperature i srčanog minutnog volumena.
Sintetski
[uredi | uredi izvor]- Amfetamin i njegovi supstituirani derivati: metamfetamin i MDMA su svi snažni hTAAR1 agonist.[13] Nakon povezivanja s TAAR1, izazivaju povećanje proizvodnje cAMP-a slično onima kod PEA i p-tiramina. Ovi spojevi su strukturno slični PEA i p-tiraminu.[10][27]
- Benzofurani: 5-APB, 5-APDB, 6-APB, 6-APDB, 4-APB, 7-APB, 5-EAPB i 5-MAPDB, kao i benzodifuran 2C-B-FLY, su hTAAR1 agonisti koji imaju farmakodinamički profil sličan MDMA.
- Metilfenetilamini su agonisti hTAAR1; oni uključuju α-metilfenetilamin (amfetamin), β-metilfenetilamin, N-metilfenetilamin (amin u tragovima), 2-metilfenetilamin, 3-metilfenetilamin i 4-metilfenetilamin.[28]
- Kod pacova, dietilamid lizergična kiselina (LSD) je agonist rTAAR1,[10] ali kod ljudi nema nikakav afinitet za hTAAR1.[28]
- Neke C 2-aminooxazoline compounds (RO5166017, RO5256390, RO5203648, i RO5263397) su oralno bioraspoloživi, visokopotentni i selektivni agonisti TAAR1 u laboratorijskih životinja.[29]
- RO5166017 ili (S) -4 -[(etilfenilamino) metil] -4,5 -dihidrooksazol -2 -ilamin je selektivni agonist TAAR1 bez značajne aktivnosti na drugim ciljevima.[30]
- RO5203648 and RO5263397 are highly selective TAAR1 partial agonists.[31] RO5203648 pokazao je jasnu antidepresivnu i antipsihotičnu aktivnost, dodatno je umanjio samoprimjenu lijekova i pokazao podsticajna svojstva budnosti i poboljšanja spoznaje u mišjim i majmunskim modelima.[32]
- Ulotaront, istraživani antipsihotik.
Parcijalni agonisti
[uredi | uredi izvor]- Ralmitaront, istraživani antipsihotik.
Inverzni agonisti
[uredi | uredi izvor]- EPPTB ili N- (3-etoksifenil) -4- (pirolidin-1-il) -3-trifluorometilbenzamid je selektivni hTAAR1 inverzni agonist.[6][33]
Neutralni antagonisti
[uredi | uredi izvor]Do početka 2018,, nisu okarakterisani neutralni antagonisti za hTAAR1.[6]
Funkcija
[uredi | uredi izvor]
Kada se amfetamin veže za TAAR1, smanjuje brzinu aktiviranja dopaminskog neurona, putem kalijevih kanala i aktivira protein kinazu A (PKA) i protein-kinazu C (PKC), koja naknadno fosforilira DAT.
PKA-fosforilacija uzrokuje povlačenje DAT-a u presinapsni neuron (internalizacija) i prestanak transporta. PKC-fosforilirani DAT može djelovati obrnuto ili, poput PKA-fosforiliranog DAT-a, internalizirati i prekinuti transport. Također je poznato da amfetamin povećava unutarstanični kalcij, što je učinak povezan s DAT fosforilacijom putem CAMKIIα-ovisnog puta, što zauzvrat proizvodi istjecanje dopamina.
Fenetilamin i amfetamin u TAAR1 lokalizirani su dopaminskim neuronima.
Monoaminski sistemi
[uredi | uredi izvor]Prije otkrića TAAR1, vjerovalo se da amini u tragovima imaju vrlo ograničene funkcije. Smatralo se da izazivaju oslobađanje noradrenalina iz simpatičkih nervnih završetaka i da se takmiče za kateholamin ili mjesta vezanja serotonina na srodnim receptorima, transporterima i mjestima skladištenja.[26] Danas se vjeruje da imaju mnogo dinamičniju ulogu u reguliranju monoaminskih sistema u mozgu.
Jedan od daljnjih efekata aktivnog TAAR1 je povećanje cikličnog adenozin-monofosfata (cAMP) u presinapsnoj ćeliji putem aktivacije Gαs G-proteina adenilil-ciklaza.[9][10][12] Samo ovo može imati mnoštvo ćelijskihih posljedica. Glavna funkcija cAMP-a može biti da poveća regulaciju ekspresije amina u tragovima u citoplazmi.[27] Ovi amini bi tada aktivirali unutarćelijski TAAR1. Monoaminski autoreceptori (npr. kratki D2, presinapsni α2 i presinapsnii 5-HT1A) imaju suprotan učinak od TAAR1, a zajedno ti receptori pružaju regulatorni sistem za monoamine.[11] Posebno, amfetamin i amini u tragovima imaju visok afinitet vezivanja za TAAR1, ali ne i za monoaminske autoreceptore.[11][13] Učinak TAAR1 agonista na monoaminske transportere u mozgu je specifičan za određeno mjesto.[11] Studije snimanja pokazuju da inhibicija ponovnog preuzimanja monoamina amfetaminom i aminama u tragovima ovisi o prisutnosti TAAR1 ko-lokalizacijom u povezanim monoaminski neuronima.[11] Od 2010., kolokalizacija TAAR1 i transportera dopamina (DAT) vizualizirana je kod rezus-majmuna, ali kolokalizacija TAAR1 sa norepinefrinskog transportera (NET) i transporterom serotonina (SERT) dokazani su samo ekspresijom iRNK.[11]
U neuronima sa kolokaliziranim TAAR1, agonisti TAAR1 povećavaju koncentracije pridruženih monoamina u sinapsnoj pukotini, čime se povećava vezivanje postsinapsnih receptora. Putem izravne aktivacije G-proteina spojenog unutrašnjeg ispravljajućeg kalijevog kanala (GIRK), TAAR1 može smanjiti brzinu okidanja dopaminskih neurona, zauzvrat sprječavajući hiper-dopaminsko stanje.[30][34][35] Amfetamin i amini u tragovima mogu ući u presinapsni neuron bilo putem dopaminskog transportera ili direktnom difuzijom kroz neuronsku membranu. Kao posljedica preuzimanja DAT-a, amfetamin i amini u tragovima proizvode konkurentnu inhibiciju ponovnog preuzimanja u transporteru.[11] Nakon ulaska u presinapsni neuron, ti spojevi aktiviraju TAAR1 koji, putem signalizacije protein-kinaza A (PKA) i protein-kinaza C (PKC), uzrokuju DAT fosforilacije. Fosforilacija bilo kojom protein-kinazom može rezultirati DAT internalizacijom (nekonkurentna inhibicija ponovnog preuzimanja), ali samo PKC-posredovana fosforilacija inducira funkciju obrnutog transportera (dopaminski efluks).[11][36]
Ekspresija TAAR1 na limfocitima povezana je s aktivacijom njihovih imunosvojstava.[16] U imunskom sistemu, TAAR1 prenosi signale kroz aktivne kaskade PKA i PKC fosforilacija.[16] U studiji iz 2012., Panas et al uočili su da je metamfetamin imao ove učinke, što ukazuje da, osim na regulaciju monoamina u mozgu, spojevi povezani s amfetaminima mogu imati uticaj na imunski sistem.[16] Nedavni rad je pokazao da je, zajedno sa TAAR1, TAAR2 potreban za punu aktivnost amina u tragovima u PMN-ćelijama.[17]
Fitohemaglutinin regulira iRNK ljudskog TAAR1-a u cirkulacijskim leukocitima;[6] u ovim ćelijama, aktivacija TAAR1 posreduje hemotaksiju leukocita prema TAAR1[6] TAAR1 agonisti (konkretno, amini u tragovima) također su pokazali da induciraju sekreciju interleukina 4 u T-ćelijama i imunoglobulina E (IgE ) u B-ćelijama.[6]
U ovim ćelijama, astrocit - lokalizirani TAAR1 regulira nivoe i funkciju EAAT2[14] što je umiješano u neuroimunski sistem izazvan metamfetaminima.[14]
Klinički značaj
[uredi | uredi izvor]Niska koncentracija fenetilamina (PEA) u mozgu povezana je s velikim depresivnim poremećajem,[9][26][37] a visoke koncentracije su povezane sa shizofrenijom.[37][38] Niski nivoi PEA i nedovoljna aktivacija TAAR1 također su povezani s ADHD.[37][38][39] Pretpostavlja se da nedovoljne razine PEA rezultiraju inaktivacijom TAAR1 i prekomjernim preuzimanjem monoamina od strane transportera, što može rezultirati depresijom.[26] Neki antidepresivi djeluju tako što inhibiraju enzim monoamin-oksidaza (MAO), koji povećava koncentraciju amina u tragovima, za koje se pretpostavlja da će povećati aktivaciju TAAR1 u presinapsnim ćelijama.[9][12] Smanjeni metabolizam PEA povezan je sa shizofrenijom, a logičan nalaz koji uzima u obzir višak PEA rezultirao bi preaktivacijom TAAR1 i sprječavanjem funkcije transportera monoamina. Mutacije u području q23.1 ljudskog hromosoma 6, istog hromosoma koji kodira TAAR1nbsp; - povezane su sa shizofrenijom.[12]
Medicinski pregledi iz februara 2015. i 2016. zabilježili su da TAAR1-selektivni ligandi imaju značajan terapijski potencijal za liječenje ovisnosti o psihostimulansima (npr. kokain, amfetamin, metamfetamin itd.).[7] Unatoč tome, široka distribucija TAAR1 izvan CNS-a i PNS-a ne utiče na krvne funkcije i regulaciju hormona štitnjače u različitim fazama starenja. Takvi podaci predstavljaju da bi buduće terapije zasnovane na TAAR1 trebale imati mali učinak u krvnoj slici i stoga će vjerojatno imati dobar sigurnosni profil.[40]
Reference
[uredi | uredi izvor]- ^ a b c GRCh38: Ensembl release 89: ENSG00000146399 - Ensembl, maj 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000056379 - Ensembl, maj 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Entrez Gene: TAAR1 trace amine associated receptor 1".
- ^ a b c d e f g h i j Maguire JJ, Davenport AP (20. 2. 2018). "Trace amine receptor: TA1 receptor". IUPHAR/BPS Guide to PHARMACOLOGY. International Union of Basic and Clinical Pharmacology. Pristupljeno 16. 7. 2018.
Tissue Distribution
CNS (region specific) & several peripheral tissues:
Stomach > amygdala, kidney, lung, small intestine > cerebellum, dorsal root ganglion, hippocampus, hypothalamus, liver, medulla oblongata, pancreas, pituitary gland, pontine reticular formation, prostate, skeletal muscle, spleen. ...
Leukocytes ...Pancreatic islet β cells ... Primary Tonsillar B Cells ... Circulating leukocytes of healthy subjects (upregulation occurs upon addition of phytohaemagglutinin).
Species: Human ...
In the brain (mouse, rhesus monkey) the TA1 receptor localises to neurones within the momaminergic pathways and there is emerging evidence for a modulatory role for TA1 on function of these systems. Co-expression of TA1 with the dopamine transporter (either within the same neurone or in adjacent neurones) implies direct/indirect modulation of CNS dopaminergic function. In cells expressing both human TA1 and a monoamine transporter (DAT, SERT or NET) signalling via TA1 is enhanced [26,48,50–51]. ...
Functional Assays ...
Mobilization of internal calcium in RD-HGA16 cells transfected with unmodified human TA1
Response measured: Increase in cytopasmic calcium ...
Measurement of cAMP levels in human cultured astrocytes.
Response measured: cAMP accumulation ...
Activation of leukocytes
Species: Human
Tissue: PMN, T and B cells
Response measured: Chemotactic migration towards TA1 ligands (β-Phenylethylamine, tyramine and 3-iodothyronamine), trace amine induced IL-4 secretion (T-cells) and trace amine induced regulation of T cell marker RNA expression, trace amine induced IgE secretion in B cells. - ^ a b Jing L, Li JX (august 2015). "Trace amine-associated receptor 1: A promising target for the treatment of psychostimulant addiction". Eur. J. Pharmacol. 761: 345–352. doi:10.1016/j.ejphar.2015.06.019. PMC 4532615. PMID 26092759.
TAAR1 is largely located in the intracellular compartments both in neurons (Miller, 2011), in glial cells (Cisneros and Ghorpade, 2014) and in peripheral tissues (Grandy, 2007) ... Existing data provided robust preclinical evidence supporting the development of TAAR1 agonists as potential treatment for psychostimulant abuse and addiction. ... Given that TAAR1 is primarily located in the intracellular compartments and existing TAAR1 agonists are proposed to get access to the receptors by translocation to the cell interior (Miller, 2011), future drug design and development efforts may need to take strategies of drug delivery into consideration (Rajendran et al., 2010).
- ^ a b c d Berry MD, Gainetdinov RR, Hoener MC, Shahid M (decembar 2017). "Pharmacology of human trace amine-associated receptors: Therapeutic opportunities and challenges". Pharmacology & Therapeutics. 180: 161–180. doi:10.1016/j.pharmthera.2017.07.002. PMID 28723415.
- ^ a b c d e f g Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S, Boyle N, Pu X, Kouranova E, Lichtblau H, Ochoa FY, Branchek TA, Gerald C (juli 2001). "Trace amines: identification of a family of mammalian G protein-coupled receptors". Proceedings of the National Academy of Sciences of the United States of America. 98 (16): 8966–8971. doi:10.1073/pnas.151105198. PMC 55357. PMID 11459929.
- ^ a b c d e Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI, Darland T, Suchland KL, Pasumamula S, Kennedy JL, Olson SB, Magenis RE, Amara SG, Grandy DK (decembar 2001). "Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor". Molecular Pharmacology. 60 (6): 1181–1188. doi:10.1124/mol.60.6.1181. PMID 11723224.
- ^ a b c d e f g h i j k l m n o Miller GM (januar 2011). "The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity". Journal of Neurochemistry. 116 (2): 164–176. doi:10.1111/j.1471-4159.2010.07109.x. PMC 3005101. PMID 21073468.
- ^ a b c d e f Lindemann L, Ebeling M, Kratochwil NA, Bunzow JR, Grandy DK, Hoener MC (mart 2005). "Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors". Genomics. 85 (3): 372–385. doi:10.1016/j.ygeno.2004.11.010. PMID 15718104.
- ^ a b c d e Grandy DK, Miller GM, Li JX (February 2016). ""TAARgeting Addiction"-The Alamo Bears Witness to Another Revolution: An Overview of the Plenary Symposium of the 2015 Behavior, Biology and Chemistry Conference". Drug Alcohol Depend. 159: 9–16. doi:10.1016/j.drugalcdep.2015.11.014. PMC 4724540. PMID 26644139.
- ^ a b c d Cisneros IE, Ghorpade A (oktobar 2014). "Methamphetamine and HIV-1-induced neurotoxicity: role of trace amine associated receptor 1 cAMP signaling in astrocytes". Neuropharmacology. 85: 499–507. doi:10.1016/j.neuropharm.2014.06.011. PMC 4315503. PMID 24950453.
TAAR1 overexpression significantly decreased EAAT-2 levels and glutamate clearance ... METH treatment activated TAAR1 leading to intracellular cAMP in human astrocytes and modulated glutamate clearance abilities. Furthermore, molecular alterations in astrocyte TAAR1 levels correspond to changes in astrocyte EAAT-2 levels and function.
- ^ Rogers TJ (2012). "The molecular basis for neuroimmune receptor signaling". J Neuroimmune Pharmacol. 7 (4): 722–724. doi:10.1007/s11481-012-9398-4. PMC 4011130. PMID 22935971.
- ^ a b c d Panas MW, Xie Z, Panas HN, Hoener MC, Vallender EJ, Miller GM (decembar 2012). "Trace amine associated receptor 1 signaling in activated lymphocytes". Journal of Neuroimmune Pharmacology. 7 (4): 866–876. doi:10.1007/s11481-011-9321-4. PMC 3593117. PMID 22038157.
- ^ a b Babusyte A, Kotthoff M, Fiedler J, Krautwurst D (mart 2013). "Biogenic amines activate blood leukocytes via trace amine-associated receptors TAAR1 and TAAR2". Journal of Leukocyte Biology. 93 (3): 387–394. doi:10.1189/jlb.0912433. PMID 23315425. S2CID 206996784.
- ^ Gainetdinov RR, Hoener MC, Berry MD (juli 2018). "Trace Amines and Their Receptors". Pharmacological Reviews. 70 (3): 549–620. doi:10.1124/pr.117.015305. PMID 29941461.
- ^ "UniProt, Q96RJ0" (jezik: engleski). Pristupljeno 11. 10. 2021.
- ^ Xie Z, Miller GM (novembar 2009). "Trace amine-associated receptor 1 as a monoaminergic modulator in brain". Biochemical Pharmacology. 78 (9): 1095–1104. doi:10.1016/j.bcp.2009.05.031. PMC 2748138. PMID 19482011.
- ^ "TAAR1". The Human Protein Atlas. Pristupljeno 24. 8. 2017.
- ^ a b c Bugda Gwilt K, González DP, Olliffe N, Oller H, Hoffing R, Puzan M, El Aidy S, Miller GM (decembar 2019). "Actions of Trace Amines in the Brain-Gut-Microbiome Axis via Trace Amine-Associated Receptor-1 (TAAR1)". Cellular and Molecular Neurobiology. 40 (2): 191–201. doi:10.1007/s10571-019-00772-7. PMID 31836967. S2CID 209339614.
- ^ Xie Z, Westmoreland SV, Bahn ME, Chen GL, Yang H, Vallender EJ, Yao WD, Madras BK, Miller GM (april 2007). "Rhesus monkey trace amine-associated receptor 1 signaling: enhancement by monoamine transporters and attenuation by the D2 autoreceptor in vitro". The Journal of Pharmacology and Experimental Therapeutics. 321 (1): 116–127. doi:10.1124/jpet.106.116863. PMID 17234900. S2CID 578835.
- ^ a b Liberles SD, Buck LB (august 2006). "A second class of chemosensory receptors in the olfactory epithelium". Nature. 442 (7103): 645–650. Bibcode:2006Natur.442..645L. doi:10.1038/nature05066. PMID 16878137. S2CID 2864195.
- ^ a b c Barak LS, Salahpour A, Zhang X, Masri B, Sotnikova TD, Ramsey AJ, Violin JD, Lefkowitz RJ, Caron MG, Gainetdinov RR (septembar 2008). "Pharmacological characterization of membrane-expressed human trace amine-associated receptor 1 (TAAR1) by a bioluminescence resonance energy transfer cAMP biosensor". Molecular Pharmacology. 74 (3): 585–594. doi:10.1124/mol.108.048884. PMC 3766527. PMID 18524885.
- ^ a b c d Zucchi R, Chiellini G, Scanlan TS, Grandy DK (decembar 2006). "Trace amine-associated receptors and their ligands". British Journal of Pharmacology. 149 (8): 967–978. doi:10.1038/sj.bjp.0706948. PMC 2014643. PMID 17088868.
Other biogenic amines are present in the central nervous system at very low concentrations in the order of 0.1–10 nm, representing <1% of total biogenic amines (Berry, 2004). For these compounds, the term ‘trace amines' was introduced. Although somewhat loosely defined, the molecules generally considered to be trace amines include para-tyramine, meta-tyramine, tryptamine, β-phenylethylamine, para-octopamine and meta-octopamine (Berry, 2004) (Figure 2).
- ^ a b Xie Z, Miller GM (juli 2009). "A receptor mechanism for methamphetamine action in dopamine transporter regulation in brain". The Journal of Pharmacology and Experimental Therapeutics. 330 (1): 316–325. doi:10.1124/jpet.109.153775. PMC 2700171. PMID 19364908.
- ^ a b Wainscott DB, Little SP, Yin T, Tu Y, Rocco VP, He JX, Nelson DL (januar 2007). "Pharmacologic characterization of the cloned human trace amine-associated receptor1 (TAAR1) and evidence for species differences with the rat TAAR1". The Journal of Pharmacology and Experimental Therapeutics. 320 (1): 475–485. doi:10.1124/jpet.106.112532. PMID 17038507. S2CID 10829497.
Several series of substituted phenylethylamines were investigated for activity at the human TAAR1 (Table 2). A surprising finding was the potency of phenylethylamines with substituents at the phenyl C2 position relative to their respective C4-substituted congeners. In each case, except for the hydroxyl substituent, the C2-substituted compound had 8- to 27-fold higher potency than the C4-substituted compound. The C3-substituted compound in each homologous series was typically 2- to 5-fold less potent than the 2-substituted compound, except for the hydroxyl substituent. The most potent of the 2-substituted phenylethylamines was 2-chloro-β-PEA, followed by 2-fluoro-β-PEA, 2-bromo-β-PEA, 2-methoxy-β-PEA, 2-methyl-β-PEA, and then 2-hydroxy-β-PEA.
The effect of β-carbon substitution on the phenylethylamine side chain was also investigated (Table 3). A β-methyl substituent was well tolerated compared with β-PEA. In fact, S-(–)-β-methyl-β-PEA was as potent as β-PEA at human TAAR1. β-Hydroxyl substitution was, however, not tolerated compared with β-PEA. In both cases of β-substitution, enantiomeric selectivity was demonstrated.
In contrast to a methyl substitution on the β-carbon, an α-methyl substitution reduced potency by ∼10-fold for d-amphetamine and 16-fold for l-amphetamine relative to β-PEA (Table 4). N-Methyl substitution was fairly well tolerated; however, N,N-dimethyl substitution was not. - ^ Galley G, Beurier A, Décoret G, Goergler A, Hutter R, Mohr S, Pähler A, Schmid P, Türck D, Unger R, Zbinden KG, Hoener MC, Norcross RD (2016). "Discovery and Characterization of 2-Aminooxazolines as Highly Potent, Selective, and Orally Active TAAR1 Agonists". ACS Med Chem Lett. 7 (2): 192–197. doi:10.1021/acsmedchemlett.5b00449. PMC 4753552. PMID 26985297.
- ^ a b Revel FG, Moreau JL, Gainetdinov RR, Bradaia A, Sotnikova TD, Mory R, Durkin S, Zbinden KG, Norcross R, Meyer CA, Metzler V, Chaboz S, Ozmen L, Trube G, Pouzet B, Bettler B, Caron MG, Wettstein JG, Hoener MC (maj 2011). "TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity". Proc. Natl. Acad. Sci. U.S.A. 108 (20): 8485–8490. Bibcode:2011PNAS..108.8485R. doi:10.1073/pnas.1103029108. PMC 3101002. PMID 21525407.
- ^ Lam VM, Espinoza S, Gerasimov AS, Gainetdinov RR, Salahpour A (juni 2015). "In-vivo pharmacology of Trace-Amine Associated Receptor 1". Eur. J. Pharmacol. 763 (Pt B): 136–42. doi:10.1016/j.ejphar.2015.06.026. PMID 26093041.
TAAR1 peripheral and immune localization/functions: It is important to note that in addition to the brain, TAAR1 is also expressed in the spinal cord (Gozal et al., 2014) and periphery (Revel et al., 2012c). It has been shown that TAAR1 is expressed and regulates immune function in rhesus monkey leukocytes (Babusyte et al., 2013; Nelson et al., 2007; Panas et al., 2012). In granulocytes, TAAR1 is necessary for chemotaxic migration of cells towards TAAR1 agonists. In addition, TAAR1 signaling in B and T cells can trigger immunoglobulin and cytokine release, respectively (Babusyte et al., 2013). TAAR1 is also expressed in the islets of Langerhans, stomach and intestines based on LacZ staining patterns carried out on TAAR1-KO LacZ mice (Revel et al., 2012c). Interestingly, the administration of selective TAAR1 partial agonist RO5263397 reverses the side effect of weight gain observed with the antipsychotic olanzapine, indicating that peripheral TAAR1 signalling can regulate metabolic homeostasis (Revel et al., 2012b). ...
Monoamine transporters and SLC22A carrier subfamily transport TAAR1 ligand: Studies using the rhesus monkey TAAR1 have shown that this receptor interacts with the monoamine transporters DAT, SERT, and NET in HEK cells (Miller et al., 2005; Xie and Miller, 2007; Xie et al., 2007). It has been hypothesized that TAAR1 interaction with these transporters might provide a mechanism by which TAAR1 ligands can enter the cytoplasm and bind to TAAR1 in intracellular compartments. A recent study has shown that in rat neonatal motor neurons, trace-amine specific signalling requires the presence and function of the transmembrane solute carrier SLC22A but not that of monoamine transporters (DAT, SERT, and NET) (Gozal et al., 2014). Specifically, it was shown that addition of β-PEA, tyramine, or tryptamine induced locomotor like activity (LLA) firing patterns of these neurons in the presence of N-Methyl D-Aspartate. Temporally, it was found that the trace amine induction of LLA is delayed compared to serotonin and norepinephrine induced LLA, indicating the target site for the trace amines is not located on the plasma membrane and could perhaps be intracellular. Importantly, blocking of SLC22A with pentamidine abolished trace amine induced LLA, indicating that trace amine induced LLA does not act on receptors found on the plasma membrane but requires their transport to the cytosol by SLC22A for induction of LLA. - ^ Revel FG, Moreau JL, Gainetdinov RR, Ferragud A, Velázquez-Sánchez C, Sotnikova TD, Morairty SR, Harmeier A, Groebke Zbinden K, Norcross RD, Bradaia A, Kilduff TS, Biemans B, Pouzet B, Caron MG, Canales JJ, Wallace TL, Wettstein JG, Hoener MC (juni 2012). "Trace Amine-Associated Receptor 1 Partial Agonism Reveals Novel Paradigm for Neuropsychiatric Therapeutics". Biol Psychiatry. 72 (11): 934–942. doi:10.1016/j.biopsych.2012.05.014. PMID 22705041. S2CID 27334223.
- ^ Bradaia A, Trube G, Stalder H, Norcross RD, Ozmen L, Wettstein JG, Pinard A, Buchy D, Gassmann M, Hoener MC, Bettler B (novembar 2009). "The selective antagonist EPPTB reveals TAAR1-mediated regulatory mechanisms in dopaminergic neurons of the mesolimbic system". Proceedings of the National Academy of Sciences of the United States of America. 106 (47): 20081–20086. Bibcode:2009PNAS..10620081B. doi:10.1073/pnas.0906522106. PMC 2785295. PMID 19892733.
- ^ Ledonne A, Berretta N, Davoli A, Rizzo GR, Bernardi G, Mercuri NB (2011). "Electrophysiological effects of trace amines on mesencephalic dopaminergic neurons". Frontiers in Systems Neuroscience. 5: 56. doi:10.3389/fnsys.2011.00056. PMC 3131148. PMID 21772817.
inhibition of firing due to increased release of dopamine; (b) reduction of D2 and GABAB receptor-mediated inhibitory responses (excitatory effects due to disinhibition); and (c) a direct TA1 receptor-mediated activation of GIRK channels which produce cell membrane hyperpolarization.
- ^ mct (28. 1. 2012). "TAAR1". GenAtlas. University of Paris. Arhivirano s originala, 29. 5. 2014. Pristupljeno 29. 5. 2014.
" • tonically activates inwardly rectifying K(+) channels, which reduces the basal firing frequency of dopamine (DA) neurons of the ventral tegmental area (VTA)" - ^ Maguire JJ, Parker WA, Foord SM, Bonner TI, Neubig RR, Davenport AP (mart 2009). "International Union of Pharmacology. LXXII. Recommendations for trace amine receptor nomenclature". Pharmacological Reviews. 61 (1): 1–8. doi:10.1124/pr.109.001107. PMC 2830119. PMID 19325074.
- ^ a b c Lindemann L, Hoener MC (maj 2005). "A renaissance in trace amines inspired by a novel GPCR family". Trends Pharmacol. Sci. 26 (5): 274–281. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
The dysregulation of TA levels has been linked to several diseases, which highlights the corresponding members of the TAAR family as potential targets for drug development. In this article, we focus on the relevance of TAs and their receptors to nervous system-related disorders, namely schizophrenia and depression; however, TAs have also been linked to other diseases such as migraine, attention deficit hyperactivity disorder, substance abuse and eating disorders [7,8,36]. Clinical studies report increased β-PEA plasma levels in patients suffering from acute schizophrenia [37] and elevated urinary excretion of β-PEA in paranoid schizophrenics [38], which supports a role of TAs in schizophrenia. As a result of these studies, β-PEA has been referred to as the body’s ‘endogenous amphetamine’ [39]
- ^ a b Sotnikova TD, Caron MG, Gainetdinov RR (august 2009). "Trace amine-associated receptors as emerging therapeutic targets". Mol. Pharmacol. 76 (2): 229–235. doi:10.1124/mol.109.055970. PMC 2713119. PMID 19389919.
Although the functional role of trace amines in mammals remains largely enigmatic, it has been noted that trace amine levels can be altered in various human disorders, including schizophrenia, Parkinson's disease, attention deficit hyperactivity disorder (ADHD), Tourette syndrome, and phenylketonuria (Boulton, 1980; Sandler et al., 1980). It was generally held that trace amines affect the monoamine system indirectly via interaction with plasma membrane transporters [such as plasma membrane dopamine transporter (DAT)] and vesicular storage (Premont et al., 2001; Branchek and Blackburn, 2003; Berry, 2004; Sotnikova et al., 2004). ...
Furthermore, DAT-deficient mice provide a model to investigate the inhibitory actions of amphetamines on hyperactivity, the feature of amphetamines believed to be important for their therapeutic action in ADHD (Gainetdinov et al., 1999; Gainetdinov and Caron, 2003). It should be noted also that the best-established agonist of TAAR1, β-PEA, shared the ability of amphetamine to induce inhibition of dopamine-dependent hyperactivity of DAT-KO mice (Gainetdinov et al., 1999; Sotnikova et al., 2004).
Furthermore, if TAAR1 could be proven as a mediator of some of amphetamine's actions in vivo, the development of novel TAAR1-selective agonists and antagonists could provide a new approach for the treatment of amphetamine-related conditions such as addiction and/or disorders in which amphetamine is used therapeutically. In particular, because amphetamine has remained the most effective pharmacological treatment in ADHD for many years, a potential role of TAAR1 in the mechanism of the “paradoxical” effectiveness of amphetamine in this disorder should be explored. - ^ Berry MD (januar 2007). "The potential of trace amines and their receptors for treating neurological and psychiatric diseases". Rev Recent Clin Trials. 2 (1): 3–19. doi:10.2174/157488707779318107. PMID 18473983.
changes in trace amines, in particular PE, have been identified as a possible factor for the onset of attention deficit/hyperactivity disorder (ADHD) [5, 27, 43, 78]. PE has been shown to induce hyperactivity and aggression, two of the cardinal clinical features of ADHD, in experimental animals [100]. Hyperactivity is also a symptom of phenylketonuria, which as discussed above is associated with a markedly elevated PE turnover [44]. Further, amphetamines, which have clinical utility in ADHD, are good ligands at trace amine receptors [2]. Of possible relevance in this aspect is modafanil, which has shown beneficial effects in ADHD patients [101] and has been reported to enhance the activity of PE at TAAR1 [102]. Conversely, methylphenidate, which is also clinically useful in ADHD, showed poor efficacy at the TAAR1 receptor [2]. In this respect it is worth noting that the enhancement of functioning at TAAR1 seen with modafanil was not a result of a direct interaction with TAAR1 [102].
More direct evidence has been obtained recently for a role of trace amines in ADHD. Urinary PE levels have been reported to be decreased in ADHD patients in comparison to both controls and patients with autism [103-105]. Evidence for a decrease in PE levels in the brain of ADHD patients has also recently been reported [4]. In addition, decreases in the urine and plasma levels of the PE metabolite phenylacetic acid and the precursors phenylalanine and tyrosine have been reported along with decreases in plasma tyramine [103]. Following treatment with methylphenidate, patients who responded positively showed a normalization of urinary PE, whilst non-responders showed no change from baseline values [105] - ^ Zhukov IS, Kubarskaya LG, Tissen IY, Kozlova AA, Dagayev SG, Kashuro VA, et al. (mart 2020). "Minimal Age-Related Alterations in Behavioral and Hematological Parameters in Trace Amine-Associated Receptor 1 (TAAR1) Knockout Mice". Cellular and Molecular Neurobiology. 40 (2): 273–282. doi:10.1007/s10571-019-00721-4. PMID 31399838. S2CID 199511689.
Vanjski linkovi
[uredi | uredi izvor]Ovaj članak uključuje tekst iz Nacionalne medicinske biblioteke Sjedinjenih Država, koji je u javnom vlasništvu.