Hypoglycin A
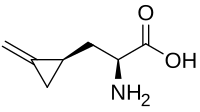
| |
| Names | |
|---|---|
| IUPAC name
(S)-2-Amino-3-((S)-2-methylenecyclopropyl)propanoic acid
| |
| Other names
Hypoglycin A; Hypoglycine; 2-Methylenecyclopropanylalanine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.189.936 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H11NO2 | |
| Molar mass | 141.170 g·mol−1 |
| Melting point | 282 °C (540 °F; 555 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hypoglycin A is a naturally occurring amino acid derivative found in the unripened fruit of the Ackee tree (Blighia sapida)[1] and in the seeds of the box elder tree (Acer negundo).[2] It is toxic if ingested, and is the causative agent of Jamaican vomiting sickness.[1] A 2017 Lancet report established a link between the consumption of unripened lychees (containing hypoglycin A or methylenecyclopropylglycine (MCPG)) resulting in hypoglycaemia and death from acute toxic encephalopathy.[3]
Sources
The entirety of the unripe Ackee fruit is toxic and contains large amounts of hypoglycin. The fruit is safe to eat only when the fruit is allowed to fully open and expose the large black seeds while on the tree. The levels of the toxin decrease over time though from approximately 1000 ppm to around 0.1 ppm in the mature fruit.[4]
Relatives of Ackee, including lychee, longan, and rambutan, can contain enough α-(methylenecyclopropyl)glycine, a homologue of hypoglycin A, in their fruit to cause hypoglycemic encephalopathy in undernourished children, when consumed in large quantities.[5]
Toxicity
Hypoglycin A is a protoxin, Meaning that the molecule is not toxic in itself but is broken down into toxic products when ingested. The branched-chain alpha-keto acid dehydrogenase complex, that normally converts leucine, isoleucine, or valine into acyl-CoA derivatives, converts Hypoglycin A into highly toxic MCPA-CoA. The FAD cofactor necessary for the beta oxidation of fatty acids associates with the alpha carbon of MCPA-CoA creating an irreversible complex that disables the enzyme. In addition, MCPA-CoA blocks some enzymes that are required for gluconeogenesis.[4]
The reduction in gluconeogenesis and the reduction in fatty acid oxidation are thought to be the cause of most of the symptoms of Jamaican vomiting sickness. The blocking of fatty acid metabolism causes cells to start using glycogen for energy. Once glycogen is depleted, the body is unable to produce more, which leads to a severe case of hypoglycemia. These biochemical effects are detected by an excess of medium chain fatty acids in urine and acidosis. Key treatments are aimed at circumventing or counteracting the biochemical changes, and include IV fluids and glucose, and hemodialysis in the case of renal failure.[6]
Synthesis
In 1958, John Carbon, William Martin, and Leo Swett were the first to synthesize hypoglycin A, in racemic form, starting from 2-bromopropene and ethyl diazoacetate to form the cyclopropane ring.[7]

See also
References
- ^ a b "Ackee Fruit Toxicity". Medscape.
- ^ Valberg, S. J.; Sponseller, B. T.; Hegeman, A. D.; Earing, J.; Bender, J. B.; Martinson, K. L.; Patterson, S. E.; Sweetman, L. (2013-07-01). "Seasonal pasture myopathy/atypical myopathy in North America associated with ingestion of hypoglycin A within seeds of the box elder tree". Equine Veterinary Journal. 45 (4): 419–426. doi:10.1111/j.2042-3306.2012.00684.x. ISSN 2042-3306.
- ^ Shrivastava, Aakash. "Association of acute toxic encephalopathy with litchi consumption in an outbreak in Muzaffarpur, India, 2014: a case-control study". The Lancet. Lancet. Retrieved 1 February 2017.
{{cite web}}: External link in|ref= - ^ a b "THE ACKEE FRUIT (BLIGHIA SAPIDA) AND ITS ASSOCIATED TOXIC EFFECTS". University of British Columbia.
- ^ Spencer, P. S.; Palmer, V. S.; Mazumder, R. (2015), "Probable Toxic Cause for Suspected Lychee-Linked Viral Encephalitis", Emerging Infectious Diseases, 21, doi:10.3201/eid2105.141650
- ^ "Hypoglycin". TOXNET.
- ^ Carbon, J. A.; Martin, W. B.; Swett, L. R. (1958), "SYNTHESIS OF α-AMINO- METHYLENECYCLOPROPANEPROPIONIC ACID (HYPOGLYCIN A)", J. Am. Chem. Soc., 80: 1002–1002, doi:10.1021/ja01537a066
