Steric effects

Steric effects are nonbonding interactions that influence the shape (conformation) and reactivity of ions and molecules. Steric effects complement electronic effects, which usually dictate shape and reactivity. Steric repulsive forces between overlapping electron clouds result in structured groupings of molecules that are stabilized by non-binding way that opposites attract and like charges repel expressed at the molecular scale.
Steric hindrance

Steric hindrance is a consequence of steric effects. Steric hindrance is the slowing of chemical reactions due to steric bulk. It is usually manifested in intermolecular reactions, whereas discussion of steric effects often focus on intramolecular interactions. Steric hindrance is often exploited to control selectivity, such as slowing unwanted side-reactions.
Steric hindrance between adjacent groups can also affect torsional bond angles. Steric hindrance is responsible for the observed shape of rotaxanes and the low rates of racemization of 2,2'-disubstituted biphenyl and binaphthyl derivatives.
Measures of steric properties
Because steric effects have profound impact on properties, the steric properties of substituents have been assessed by numerous methods.
Rate data
Relative rates of chemical reactions provide useful insights into the effects of the steric bulk of substituents. Under standard conditions methyl bromide solvolyzes 107 faster than does neopentyl bromide. The difference reflects the inhibition of attack on the compound with the sterically bulky (CH3)3C group.[3]
A-values
A values provide another measure of the bulk of substituents. A values are derived from equilibrium measurements of monosubstituted cyclohexanes.[4][5][6][7] The extent that a substituent favors the equatorial position gives a measure of its bulk.

| Substituent | A-Value |
|---|---|
| H | 0 |
| CH3 | 1.74 |
| CH2CH3 | 1.75 |
| CH(CH3)2 | 2.15 |
| C(CH3)3 | >4 |
Ceiling temperatures
Ceiling temperature () is a measure of the steric properties of the monomers that comprise a polymer. is the temperature where the rate of polymerization and depolymerization are equal. Sterically hindered monomers give polymers with low 's, which are usually not useful.
| Monomer | Ceiling temperature (°C)[8] | Structure |
|---|---|---|
| ethylene | 610 | CH2=CH2 |
| isobutylene | 175 | CH2=CMe2 |
| 1,3-butadiene | 585 | CH2=CHCH=CH2 |
| isoprene | 466 | CH2=C(Me)CH=CH2 |
| styrene | 395 | PhCH=CH2 |
| α-methylstyrene | 66 | PhC(Me)=CH2 |
Cone angles

| Ligand | Angle (°) |
|---|---|
| PH3 | 87 |
| P(OCH3)3 | 107 |
| P(CH3)3 | 118 |
| P(CH2CH3)3 | 132 |
| P(C6H5)3 | 145 |
| P(cyclo-C6H11)3 | 179 |
| P(t-Bu)3 | 182 |
| P(2,4,6-Me3C6H2)3 | 212 |
Ligand cone angles are measures of the size of ligands in coordination chemistry. It is defined as the solid angle formed with the metal at the vertex and the hydrogen atoms at the perimeter of the cone (see figure).[9]
Significance and applications
Steric effects are critical to chemistry, biochemistry, and pharmacology. In organic chemistry, steric effects are nearly universal and affect the rates and activation energies of most chemical reactions to varying degrees.
In biochemistry, steric effects are often exploited in naturally occurring molecules such as enzymes, where the catalytic site may be buried within a large protein structure. In pharmacology, steric effects determine how and at what rate a drug will interact with its target bio-molecules.
- Prominent Sterically Hindered Compounds
-
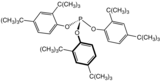
Tris(2,4-di-tert-butylphenyl)phosphite, a widely used stabilizer in polymers.
-
Tricyclohexylphosphine, a bulky phosphine ligand used in homogeneous catalysis and, with B(C6F5)3, comprises the classic frustrated Lewis pair.[10]
-
2,6-Di-tert-butylphenol is used industrially as UV stabilizers and antioxidants for hydrocarbon-based products ranging from petrochemicals to plastics.[11]
-
Titanium isopropoxide is a monomer, the corresponding titanium ethoxide is a tetramer.
-
An isolable selenenic acid owing to steric protection.[14]

See also
- Collision theory
- Reaction rate accelerate as result of steric hindrance in the Thorpe–Ingold effect
- Sterically induced reduction
- Intramolecular force
- Van der Waals strain, also known as steric strain
References
- ^ "Tetra-tert-butyltetrahedrane". Angew. Chem. Int. Ed. Engl. 17: 520–1. 1978. doi:10.1002/anie.197805201.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Gait, Michael (1984). Oligonucleotide synthesis: a practical approach. Oxford: IRL Press. ISBN 0-904147-74-6.
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1
- ^ E.L. Eliel, S.H. Wilen and L.N. Mander, Stereochemistry of Organic Compounds, Wiley, New York (1994). ISBN 81-224-0570-3
- ^ Eliel, E.L.; Allinger, N.L.; Angyal, S.J.; G.A., Morrison (1965). Conformational Analysis. New York: Interscience Publishers.
- ^ Hirsch, J.A. (1967). Topics in Stereochemistry (first ed.). New York: John Wiley & Sons,Inc. p. 199.
- ^ Romers, C.; Altona, C.; Buys, H.R.; Havinga, E. (1969). Topics in Stereochemistry (fourth ed.). New York: John Wiley & Sons,Inc. p. 40.
- ^ Stevens, Malcolm P. (1999). "6". Polymer Chemistry an Introduction (3rd ed.). New York: Oxford University Press. pp. 193–194. ISBN 978-0-19-512444-6.
- ^ Tolman, Chadwick A. (1970-05-01). "Phosphorus ligand exchange equilibriums on zerovalent nickel. Dominant role for steric effects". J. Am. Chem. Soc. 92 (10): 2956–2965. doi:10.1021/ja00713a007.
- ^ Stephan, Douglas W. "Frustrated Lewis pairs": a concept for new reactivity and catalysis. Org. Biomol. Chem. 2008, 6, 1535-1539. doi:10.1039/b802575b
- ^ "Phenol Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. 2002. doi:10.1002/14356007.a19_313.
{{cite encyclopedia}}: Unknown parameter|authors=ignored (help) - ^ Norman S. Allen, ed. (2010). "Photostabilisation of Polymer Materials". Photochemistry and Photophysics of Polymer Materials Photochemistry. Hoboken: John Wiley & Sons. doi:10.1002/9780470594179.ch17.
{{cite book}}: Unknown parameter|authors=ignored (help). - ^ Klaus Köhler; Peter Simmendinger; Wolfgang Roelle; Wilfried Scholz; Andreas Valet; Mario Slongo (2010). "Paints and Coatings, 4. Pigments, Extenders, and Additives". Ullmann's Encyclopedia Of Industrial Chemistry. doi:10.1002/14356007.o18_o03.
- ^ Goto, Kei; Nagahama, Michiko; Mizushima, Tadashi; Shimada, Keiichi; Kawashima, Takayuki; Okazaki, Renji (2001). "The First Direct Oxidative Conversion of a Selenol to a Stable Selenenic Acid: Experimental Demonstration of Three Processes Included in the Catalytic Cycle of Glutathione Peroxidase". Organic Letters. 3 (22): 3569–3572. doi:10.1021/ol016682s. PMID 11678710.
External links
- Steric Effects (chem.swin.edu.au) at the Wayback Machine (archived July 25, 2008)
- Steric: A Program to Calculate the Steric Size of Molecules (gh.wits.ac.za) at the Wayback Machine (archived December 22, 2017)


![Tricyclohexylphosphine, a bulky phosphine ligand used in homogeneous catalysis and, with B(C6F5)3, comprises the classic frustrated Lewis pair.[10]](/media/wikipedia/commons/thumb/e/eb/Tricyclohexylphosphine-2D-skeletal.png/157px-Tricyclohexylphosphine-2D-skeletal.png)
![2,6-Di-tert-butylphenol is used industrially as UV stabilizers and antioxidants for hydrocarbon-based products ranging from petrochemicals to plastics.[11]](/media/wikipedia/commons/thumb/0/06/2%2C6-di-tert-butylphenol.svg/157px-2%2C6-di-tert-butylphenol.svg.png)
![Hindered amine light stabilizers are widely used in polymers.[12][13]](/media/wikipedia/commons/thumb/5/57/LMW-HA%28L%29S-1_100.svg/160px-LMW-HA%28L%29S-1_100.svg.png)

![An isolable selenenic acid owing to steric protection.[14]](/media/wikipedia/commons/thumb/4/4c/OkazakiRSeOH.png/128px-OkazakiRSeOH.png)