Isotoluene

The isotoluenes in organic chemistry are the non-aromatic toluene isomers with an exocyclic double bond. They are of some academic interest in relation to aromaticity and isomerisation mechanisms.[1][2]
The three basic isotoluenes are ortho-isotoluene or 5-methylene-1,3-cyclohexadiene (here labelled 1); para-isotoluene (2); and meta-isotoluene (3). Another structural isomer is the bicyclic compound 5-methylenebicyclo[2.2.0] hexene (4).
The o- and p-isotoluenes isomerise to toluene, a reaction driven by aromatic stabilisation. It is estimated that these compounds are 96 kJ mol−1 less stable.
The isomerisation of p-isotoluene to toluene takes place at 100 °C in benzene with bimolecular reaction kinetics by an intermolecular free radical reaction. The intramolecular isomerisation, a 1,3-sigmatropic reaction, is unfavorable because an antarafacial mode is enforced.[3] Other dimer radical reaction products are formed as well.
The ortho-isomer is found to isomerise at 60 °C in benzene, also in a second order reaction. The proposed reaction mechanism is a concerted intermolecular ene reaction. The reaction product is either toluene or a mixture of dimerized ene reaction products, depending on the exact reaction conditions.
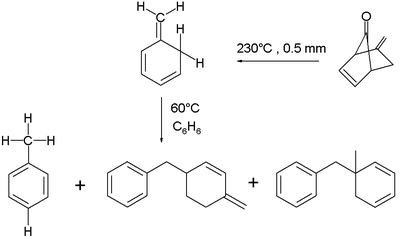
Ortho-isotoluene has been researched in connection with the mechanism of initiator-free polymerization of polystyrene.
See also
References
- ^ Radical production from the interaction of closed-shell molecules. 10. Chemistry of methylenecyclohexadiene and the thermal polymerization of styrene W. David Graham, John Glass Green, and William A. Pryor J. Org. Chem.; 1979; 44(6) pp 907 - 914; doi:10.1021/jo01320a003.
- ^ Bimolecular reactions of 3-methylene-1,4-cyclohexadiene (p-isotoluene), 5-methylene-1,3-cyclohexadiene (o-isotoluene), 1-methylene-1,4-dihydronaphthalene (benzo-p-isotoluene), and 9-methylene-9,10-dihydroanthracene (dibenzo-p-isotoluene) Joseph J. Gajewski and Andrea M. Gortva J. Org. Chem.; 1989; 54(2) pp 373 - 378; doi:10.1021/jo00263a021
- ^ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 3rd edition, New York: Wiley, ISBN 9780471854722, OCLC 642506595
