| Properties of water | |
|---|---|
| |
| General | |
| Systematic name | Water |
| Other names | Aqua |
| Molecular formula | H2O |
| Molar mass | 18.01528 g/mol |
| Appearance | transparant, almost colourless liquid with a slight hint of blue [1] |
| CAS number | [7732-18-5] |
| Properties | |
| Density and phase | 1 g/cm3, liquid |
| Melting point | 0 °C (273.15 K) |
| Boiling point | 100 °C (373.15 K) |
| Acidity (pKa) | 7.0 |
| Basicity (pKb) | 7.0 |
| Viscosity | 1 mPa·s at 20 °C |
| Structure | |
| Molecular shape | non-linear bent |
| Crystal structure | ? |
| Dipole moment | ? D |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | No known hazard |
| NFPA 704 | |
| R/S statement | R: - S: 36/37, 39 |
| RTECS number | ZC0110000 |
| Supplementary data page | |
| Structure & properties | n, εr, etc. |
| Thermodynamic data | Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Related solvents | acetone methanol |
| Related compounds | ice DHMO heavy water |
| Except where noted otherwise, data are given for materials in their standard state (at 25°C, 100 kPa) Infobox disclaimer and references | |
Water is in dynamic equilibrium between the liquid and solid states at standard temperature and pressure. At room temperature, approximately 25°C, it is a liquid. It has the chemical formula H2O, meaning that one molecule of water is composed of two hydrogen atoms and one oxygen atom.
Forms of water
- See the Category:Forms of water
Water takes many forms. The solid state of water is known as ice; the gaseous state is known as water vapor (or steam). Water has many other forms, such as vitreous ice, a noncrystalline (glassy), solid state of water.
Above a certain critical temperature and pressure (647 K and 22.064 MPa), water molecules assume a supercritical condition, in which liquid-like clusters float within a vapor-like phase.
Heavy water is water in which the hydrogen atoms are replaced by its heavier isotope, deuterium. It is chemically the same as normal water. Heavy water is used in the nuclear industry to slow down neutrons.
A common substance
Water in the Universe
Water has been found in interstellar clouds within our galaxy, the Milky Way. It is believed that water exists in abundance in other galaxies too, because its components, hydrogen and oxygen, are among the most abundant elements in the universe.
Interstellar clouds eventually condense into solar nebulae and solar systems, such as ours. The initial water can then be found in comets, planets, and their satellites. In our solar system, water, in liquid or ice form, has been found :
- on the Moon,
- on the planets Mercury, Mars, Neptune, and Pluto,
- on satellites of planets, such as Triton and Europa.
Water on Earth
The water cycle (known scientifically as the hydrologic cycle) refers to the continuous exchange of water within the hydrosphere, between the atmosphere, soil water, surface water, groundwater, and plants.
Earth's approximate water volume (the total water supply of the world) is 1,360,000,000 km³ (326,000,000 mi³). Of this volume:
- 1,320,000,000 km³ (316,900,000 mi³ or 97.2%) is in the oceans
- 25,000,000 km³ (6,000,000 mi³ or 1.8%) is in glaciers and icecaps
- 13,000,000 km³ (3,000,000 mi³ or 0.9%) is groundwater.
- 250,000 km³ (60,000 mi³ or 0.02%) is fresh water in lakes, inland seas, and rivers.
- 13,000 km³ (3,100 mi³ or 0.001%) is atmospheric water vapor at any given time.
Liquid water is found in bodies of water, such as an ocean, sea, lake, river, stream, canal, or pond. The majority of water on Earth is sea water. Water is also present in the atmosphere in both liquid and vapor phases. It also exists as groundwater in aquifers.
Water in industry
Water is also used in many industrial processes and machines, such as the steam turbine and heat exchanger, in addition to its use as a chemical solvent. Discharge of untreated water from industrial uses is pollution. Pollution includes discharged solutes (chemical pollution) and discharged coolant water (thermal pollution). Industry requires pure water for many applications and utilizes a variety of purification techniques both in water supply and discharge.
Physics and chemistry of water
Density of water and ice
For most substances, the solid form of the substance is more dense than the liquid form; thus, a block of pure solid will sink in a tub of pure liquid. But a block of ice will float in a tub of water because solid water is less dense than liquid water. This is the first unusual property of water. At room temperature, liquid water becomes denser with lowering temperature, just like other substances. But at 4°C, just above freezing, water reaches its maximum density, and as water cools further toward freezing, the liquid water expands to become less dense. The physical reason for this is the related to the crystal structure of ordinary ice, known as hexagonal ice Ih. Water, gallium, and bismuth are some of the few materials which expand when they freeze; most other materials contract.
This unusual expansion of water as it cools from 4°C above freezing to the freezing point offers an important advantage for freshwater life in winter. Water chilled at the surface becomes denser and sinks, forming convection currents that cool the whole water body, but when the temperature of the lake water reaches 4°C, water on the surface, as it chills further, becomes less dense, and stays as a surface layer which eventually forms ice. Since downward convection of colder water is blocked by the density change, any large body of fresh water frozen in winter will have the coldest water near the surface, away from the riverbed or lakebed.
Density of saltwater and ice
The situation in salt water is somewhat different. Ice still floats to keep the oceans from freezing solid (see following paragraph). However, the salt content of oceans both lowers the freezing point by about 2°C and lowers the temperature of the density maximum of water to be about at the freezing point. Hence, in ocean water because of the salt content, the downward convection of colder water is not blocked by an expansion of water as it becomes colder near the freezing point; thus the oceans' cold water near the freezing point continues to sink. For this reason, any creature attempting to survive at the bottom of such cold water as the Arctic Ocean is generally lives in water that is 4°C colder than the temperature at the bottom of frozen-over fresh water lakes and rivers in winter.
As the surface of salt water begins to freeze (at -1.9°C for normal salinity seawater, 35‰) the ice that forms is essentially salt free with a density approximately that of freshwater ice. This ice floats on the surface and the salt that is "frozen out" adds to the salinity and density of the seawater subjacent to it. This more dense saltwater sinks by convection and the replacing seawater is subject to the same process. This provides essentially freshwater ice at -1.9°C on the surface. The increased density seawater beneath the forming ice sinks toward the bottom, thus the deep ocean waters should have a minimum temperature of −1.9°C also.
Triple point
The temperature and pressure at which solid, liquid, and gaseous water coexist in equilibrium is called the triple point of water. This point is used to define the units of temperature (the kelvin and, indirectly, the degree Celsius and even the degree Fahrenheit). The triple point is at a temperature of 273.16 K (0.01 °C) by convention, and at a pressure of 611.2 Pa. This pressure is quite low and a pretty good vacuum, normal sea level barometric pressure is about 101,300 Pa for comparison.
Mpemba effect
The Mpemba effect is the surprising phenomenon whereby hot water can, under certain conditions, freeze faster than cold, even though it must pass the lower temperature on the way to freezing. However, this can be explained with evaporation, convection, supercooling, and the insulating effect of frost.
Surface tension
Water drops are stable thanks to the high surface tension of water. This can be seen when small quantities of water are put onto a nonsoluble surface such as glass: the water stays together as drops. This property is important for life. For an example when water is carried through xylem up stems in plants the strong intermolecular attractions hold the water column together. Strong cohesive properties hold the water column together, and strong adhesive properties stick the water to the xylem, and prevent tension rupture caused by transpiration pull. Other liquids with lower surface tension would have a higher tendency to "rip", forming vacuum or air pockets and rendering the xylem water transport inoperative.
Electrical properties
Pure water is actually a good insulator (poor conductor), meaning that it does not conduct electricity well. Because water is such a good solvent, however, it almost always has some solute dissolved in it, most frequently a salt. If water has even a tiny amount of such impurities, then it can conduct electricity much better, because impurities such as salt separate into free ions in aqueous solution by which an electric current can flow.
Water can be split into its constituent elements, hydrogen and oxygen, by passing a current through it. This process is called electrolysis. Water molecules naturally dissociate into H+ and OH- ions, which are pulled toward the cathode and anode, respectively. At the cathode, two H+ ions pick up electrons and form H2 gas. At the anode, four OH- ions combine and release O2 gas, molecular water, and four electrons. The gases produced bubble to the surface, where they can be collected. It is known that the theoretical maximum electrical resistivity for water is approximately 182 kilohm-meters (or 18.2 MΩ·cm) at 25 degrees Celsius. This figure agrees well with what is typically seen on reverse osmosis, ultrafiltered and deionized ultrapure water systems used for instance, in semiconductor manufacturing plants. A salt or acid contaminant level exceeding that of even 100 parts per trillion (ppt) in ultrapure water will begin to noticeably lower its resistivity level by up to several kilohm-meters (a change of several hundred kilosiemens per meter of conductance).
Magnetic property
Since its molecules have no intrinsic magnetic moment, water is diamagnetic, meaning it is weakly repelled by strong magnets. As with most diamagnetic substances, this effect is extremely small, with χm = −9.0 ×10-6 (μ = 1.2566258 µN/A²).
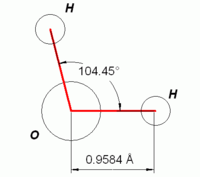
Dipolar nature of water
An important feature of water is its polar nature. The water molecule forms an angle, with hydrogen atoms at the tips and oxygen at the vertex. Since oxygen has a higher electronegativity than hydrogen, the side of the molecule with the oxygen atom has a partial negative charge. A molecule with such a charge difference is called a dipole. The charge differences cause water molecules to be attracted to each other (the relatively positive areas being attracted to the relatively negative areas) and to other polar molecules. This attraction is known as hydrogen bonding, and explains many of the properties of water.
Although hydrogen bonding is a relatively weak attraction compared to the covalent bonds within the water molecule itself, it is responsible for a number of water's physical properties. One such property is its relatively high melting and boiling point temperatures; more heat energy is required to break the hydrogen bonds between molecules. The similar compound hydrogen sulfide (H2S), which has much weaker hydrogen bonding, is a gas at room temperature even though it has twice the molecular weight of water. The extra bonding between water molecules also gives liquid water a large specific heat capacity. This high heat capacity makes water a good heat storage medium.
Hydrogen bonding also gives water its unusual behavior when freezing. When cooled to near freezing point, the presence of hydrogen bonds means that the molecules, as they rearrange to minimize their energy, form the hexagonal crystal structure of ice that is actually of lower density: hence the solid form, ice, will float in water. In other words, water expands as it freezes, whereas virtually all other materials shrink on solidification.
An interesting consequence of the solid having a lower density than the liquid is that ice will melt if sufficient pressure is applied. With increasing pressure the melting point temperature drops and when the melting point temperature is lower than the ambient temperature the ice begins to melt. A significant increase of pressure is required to lower the melting point temperature by very much - the pressure exerted by an ice skater on the ice would only reduce the melting point by something like 0.09 °C.
Water as a solvent
Water is also a good solvent due to its polarity. When an ionic or polar compound enters water, it is surrounded by water molecules. The relatively small size of water molecules typically allows many water molecules to surround one molecule of solute. The partially negative dipole ends of the water are attracted to positively charged components of the solute, and vice versa for the positive dipole ends.
In general, ionic and polar substances such as acids, alcohols, and salts are relatively soluble in water, and nonpolar substances such as fats and oils are not. Nonpolar molecules stay together in water because it is energetically more favorable for the water molecules to hydrogen bond to each other than to engage in van der Waals interactions with nonpolar molecules.
An example of an ionic solute is table salt; the sodium chloride, NaCl, separates into Na+ cations and Cl- anions, each being surrounded by water molecules. The ions are then easily transported away from their crystalline lattice into solution. An example of a nonionic solute is table sugar. The water dipoles make hydrogen bonds with the polar regions of the sugar molecule (OH groups) and allow it to be carried away into solution.
The solvent properties of water are vital in biology, because many biochemical reactions take place only within aqueous solutions (e.g., reactions in the cytoplasm and blood).
Amphoteric nature of water
Chemically, water is amphoteric -- i.e., it is able to act as either an acid or a base. Occasionally the term hydroxic acid is used when water acts as an acid in a chemical reaction. At a pH of 7 (neutral), the concentration of hydroxide ions (OH-) is equal to that of the hydronium (H3O+) or hydrogen (H+) ions. If the equilibrium is disturbed, the solution becomes acidic (higher concentration of hydronium ions) or basic (higher concentration of hydroxide ions).
Water can act as either an acid or a base in reactions. According to the Brønsted-Lowry system, an acid is defined as a species which donates a proton (an H+ ion) in a reaction, and a base as one which receives a proton. When reacting with a stronger acid, water acts as a base; when reacting with a weaker acid, it acts as an acid. For instance, it receives an H+ ion from HCl in the equilibrium:
- HCl + H2O ↔ H3O+ + Cl-
Here water is acting as a base, by receiving an H+ ion. An acid donates an H+ ion, and water can also do this, such as in the reaction with ammonia, NH3:
- NH3 + H2O ↔ NH4+ + OH-
Acidity in nature
In theory, pure water has a pH of 7. In practice, pure water is very difficult to produce. Water left exposed to air for any length of time will rapidly dissolve carbon dioxide, forming a dilute solution of carbonic acid, with a limiting pH of about 5.7. As cloud droplets form in the atmosphere and as raindrops fall through the air minor amounts of CO2 are absorbed and thus most rain is slightly acid. If high amounts of nitrogen and sulfur oxides are present in the air, they too will dissolve into the cloud and rain drops producing more serious acid rain problems.
Hydrogen bonding in water
Water molecule can form a maximum of four hydrogen bonds because it can accept two and donate two hydrogens. Other molecules like hydrogen fluoride, ammonia, methanol form hydrogen bonds but they do not show anomalous behaviour of thermodynamic, kinetic and structural properties like those observed in water. The answer to the apparent difference between water and other hydrogen bonding liquids lies in the fact that apart from water none of the hydrogen bonding molecules can form four hydrogen bonds either due to inability to donate/accept hydrogens or steric effect due to bulky residue. In water local tetrahedral order arising due to the four hydrogen bonds gives rise to open structure and 3-dimensional network in contrast to close packed structure of simple liquids. There is a great similarity between water and silica in their anomalous behaviour although one has hydrogen bonding network and the other has a network made of ionic bonds with partial covalent character. But what makes water the chosen one for life-forms is that it shows the special properties over a temperature regime which suits different biological processes including hydration.
It is believed that hydrogen bond in water is largely due to electrostatic forces and some amount of covalency. The partial covalent nature of hydrogen bond predicted by Linus Pauling in 1930s is yet be to proven unambiguously by experiments and theoretical calculations.
History
In 1742, Anders Celsius defined the Celsius temperature scale with the freezing point of water at 100 degrees and the boiling point at standard atmospheric pressure at 0 degrees. The scale was reversed in 1744.
The first decomposition of water into hydrogen and oxygen, by electrolysis, was done in 1800 by William Nicholson (chemist), an English chemist.
Gilbert Newton Lewis isolated the first sample of pure heavy water in 1933.
Polywater was a hypothetical polymerized form of water that was the subject of much scientific controversy during the late 1960s. The consensus now is that it does not exist.
Systematic nomenclature and humor
Chemists sometimes jokingly refer to water as dihydrogen monoxide or DHMO, an overly pedantic systematic covalent name of this molecule, especially in parodies of chemical research that call for this "lethal chemical" to be banned. In 2004, the town of Aliso Viejo, California nearly banned foam cups after learning that DHMO was used in their production (see [2]). In reality, a more realistic systematic name would be hydrogen oxide, since the "di-" and "mon-" prefixes are superfluous. Hydrogen sulfide, H2S, is never referred to as "dihydrogen monosulfide", and hydrogen peroxide, H2O2, is never called "dihydrogen dioxide".
Some overzealous MSDSs for water list the following: Caution: May cause drowning!
The systematic acid name of water is hydroxic acid or hydroxilic acid. Likewise, the systematic alkali name of water is hydrogen hydroxide – both acid and alkali names exist for water because it is able to react both as an acid or an alkali, depending on the strength of the acid or alkali it is reacted with (it is amphoteric). None of these names is used widely outside of DHMO sites.