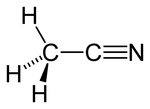
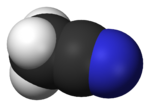
Acetonitrile
Acetonitrile is the chemical compound with formula of CH3CN. This colourless liquid is the simplest organic nitrile and is widely used as a solvent.
Industrial applications
Acetonitrile is used as a solvent but also as an intermediate in the production of many chemicals ranging from pesticides to perfumes. Production trends for acetonitrile generally follow those of acrylonitrile. The four main producers of acetonitrile in the United States are: BP Chemicals, DuPont, J.T. Baker Chemical, and Sterling Chemicals. In 1992, 32.3 million pounds (14,700 t) of acetonitrile were produced in the U.S.
Laboratory uses
Acetonitrile is commonly the solvent of choice for testing an unknown chemical reaction. It is polar with a convenient liquid range. It dissolves a wide range of chemical compounds without complications due to the presence of acidic protons. Acetone has similar properties but is more acidic and more reactive toward bases and nucleophiles. (Note: the pKa given applies to the conjugate acid of acetonitrile, not the molecule itself.)
In inorganic chemistry, acetonitrile is widely employed as a dispaceable ligand where it is abbreviated MeCN. A good example is the use of PdCl2(MeCN)2 prepared by refluxing polymeric palladium chloride in acetonitrile to obtain a solution of more reactive molecular palladium species.
It is a popular solvent in cyclic voltammetry because of its relatively high dielectric constant. MeCN is a two-carbon building block in organic synthesis.[1] Acetonitrile is also commonly used in column chromatography and the more modern high performance liquid chromatography where it serves as a mobile phase in the separation of molecules.
Safety
Acetonitrile is toxic and flammable. It is metabolized into hydrogen cyanide and thiocyanate.[citation needed]
External links
- International Chemical Safety Card 0088
- National Pollutant Inventory - Acetonitrile fact sheet
- NIOSH Pocket Guide to Chemical Hazards
References
- ^ DiBiase, S. A.; Beadle, J. R.; Gokel, G. W. “Synthesis of α,β-Unsaturated Nitriles from Acetonitrile: Cyclohexylideneacetonitrile and Cinnamonitrile” Organic Syntheses, Collective Volume 7, page 108.