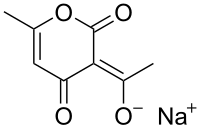
Sodium dehydroacetate
| |
| Names | |
|---|---|
| Other names
E266
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.022.347 |
| E number | E266 (preservatives) |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H7NaO4 | |
| Molar mass | 190.130 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium dehydroacetate is a compound with the formula Na(CH3C5HO(O2)(CH3)CO). It is the sodium salt of dehydroacetic acid. It is used as a preservative in food,[1] cosmetics and personal care products such as shower gels, which is highly effective against a broad range of bacteria even at low concentrations such as 0.075 g/kg in meat. Its use is regulated in China, Japan and the USA, thus it can only be used in certain foods such as picked vegetables, fermented soy, compound seasonings and pickled mushrooms in China, and only peeled pumpkins in the US at 65 parts per million. It is not approved for use in the EU. It adds an acetic odor to food which can be mitigated using flavorings.[2] It can also be used in bread and pastries.[3] It is highly effective when applied to oranges.[4]
It has E number E266.
References
[edit]- ^ Li, Lu; Tang, Xu; Ouyang, Qiuli; Tao, Nengguo (2019). "Combination of sodium dehydroacetate and sodium silicate reduces sour rot of citrus fruit". Postharvest Biology and Technology. 151: 19–25. doi:10.1016/j.postharvbio.2019.01.006.
- ^ "Food Additives Gradually Going Down the Drain: Sodium Dehydroacetate (E 266)". ZMUni Compliance Centre. November 30, 2022.
- ^ Han, James (July 20, 2020). "What is Sodium Dehydroacetate in food: Uses and Safety". foodadditives.net.
- ^ Li, Lu; Tang, Xu; Ouyang, Qiuli; Tao, Nengguo (May 2019). "Combination of sodium dehydroacetate and sodium silicate reduces sour rot of citrus fruit". Postharvest Biology and Technology. 151: 19–25. doi:10.1016/j.postharvbio.2019.01.006.
