Halogen lamp
A halogen lamp (also called tungsten-halogen lamp, quartz-halogen lamp, or quartz-iodine lamp) is an incandescent lamp wherein a tungsten filament is sealed into a small transparent envelope filled with a halogen gas such as iodine or bromine. The halogen lamp can operate its filament at a higher temperature than in a standard vacuum or inert-gas filled lamp, without loss of operating life. This gives it a slightly higher efficiency (10-20%) than standard incandescent lamps. It gives a bright yellow light that is considered by many to be the nearest to daylight from any lamp.

Principle of operation

In an ordinary incandescent lamp, the thickness of the filament may vary slightly. Although the resistance of the filament is higher at the thinner portions, the current is the same. Therefor the thin areas are hotter than the thicker parts of the filament. The rate of tungsten evaporation will be higher at these points due to the increased temperature, causing the thin areas to become even thinner, creating a runaway effect until the filament fails. A tungsten-halogen lamp creates an equilibrium reaction in which the tungsten that evaporates when giving off light is preferentially re-deposited at the hot-spots, preventing the early failure of the lamp. This chemical transport reaction is based on tungsten halides, WI2 or WBr2, formed by the reaction of evaporated tungsten with halogen added to the gas filling of the bulb. The tungsten halides decompose at the hot tungsten filament, redepositing elemental tungsten onto the filament.[1]
This also allows halogen lamps to be run at higher temperatures which would cause unacceptably short lamp lifetimes in ordinary incandescent lamps, allowing for higher luminous efficacy, apparent brightness, and whiter color temperature. Because the lamp must be very hot to prevent condensation of WI2 or WBr2 at the glass surface, the halogen lamp's envelope must be made of hard glass or fused quartz, instead of ordinary soft glass which would soften and flow too much at these temperatures.
The envelope material can be selected and modified (by means of optical coating) to achieve whatever lamp characteristics are required. Halogen bulbs are widely used in automobile headlamps, for example, and because headlamps often contain plastic parts, halogen headlamp bulbs' envelopes are made out of hard glass, or out of quartz 'doped' with additives to block most of the UV output (hard glass blocks UV without need of dopants).
Conversely, some applications require ultraviolet radiation, and in such cases, the lamp envelope is made out of undoped quartz. Thus, the lamp becomes a source of UV-B radiation. Undoped quartz halogen lamps are used in some scientific, medical and dental instruments as a UV-B source
A typical halogen lamp is designed to run for about 2000 hours, twice as long as a typical incandescent lamp.
Halogen infrared
A further development that has added to halogen lamp efficacy is an infrared-reflective coating (IRC). The quartz envelope is coated with a multi-layered dichroic coating which allows visible light to be emitted while reflecting a portion of the infrared radiation back onto the filament. Such lamps are called halogen-infrared lamps, and they require less power than standard halogen lamps to produce any given light output. The efficiency increase can be as much as 40% when compared to its standard equivalent. Harison Toshiba Lighting Corporation (Imabari, Japan) manufactures a halogen infrared bulb called HIR-1 for automotive headlamp use that achieves 2,500 lumens out of 65 watts input power (38 lumens per watt); this bulb uses IR-reflective coatings licensed from General Electric[2].
Halogen Lamp Spectra
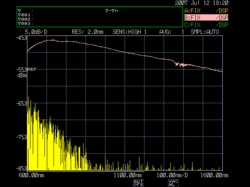
A Halogen incandescent lamp has a light spectrum as shown in the accompanying figure. The figure shows just the infrared end of the whole spectrum, which is limited by the resolution of the spectrum analyzer from 600nm to 1500nm, in the optical window, typical to fiber optic communication systems.
Safety
Because the halogen lamp operates at very high temperatures, it can pose fire and burn hazards. Additionally, it is possible to get a sunburn from excess exposure to the UV emitted by an undoped quartz halogen lamp. To reduce unintentional UV exposure, and to contain hot bulb fragments in the event of explosive bulb failure, general-purpose lamps usually have a UV-absorbing glass filter over or around the bulb. Alternatively, lamp bulbs may be coated to filter out the UV radiation. When this is done correctly, a halogen lamp with UV inhibitors will produce less UV than its standard incandescent counterpart.
Handling precautions
Any surface contamination, notably fingerprints, can damage the quartz envelope when it is heated. Contaminants will create a hot spot on the bulb surface when the bulb is turned on. This extreme, localized heat causes the quartz to change from its vitreous form into a weaker, crystalline form which leaks gas. This weakening may also cause the bulb to rapidly form a bubble, thereby weakening the bulb and leading to its failure or explosion, and creating a serious safety hazard. Consequently, quartz lamps should be handled without ever touching the clear quartz, either by using a clean paper towel or carefully holding the porcelain base. If the quartz is contaminated in any way, it must be thoroughly cleaned with rubbing alcohol and dried before use.
References
- ^ see e.g. OSRAM-SYLVANIA information about tungsten halogen lamps
- ^ "Halogen Forward-lighting Bulb with IR-Reflective Films". Harison Toshiba Lighting Corp. 1997–2007. Retrieved 2007-05-02.
{{cite web}}: CS1 maint: date format (link)
