Shape-memory alloy
A shape memory alloy (SMA, also known as a smart alloy, memory metal, or muscle wire) is an alloy that "remembers" its shape.
Overview
This article needs attention from an expert on the subject. Please add a reason or a talk parameter to this template to explain the issue with the article. |
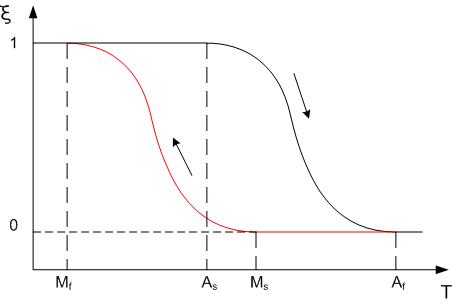
The three main types of SMA are the copper-zinc-aluminum-nickel, copper-aluminum-nickel, and nickel-titanium (NiTi) alloys. NiTi alloys are generally more expensive and to change from austenite to martensite upon cooling; Mf is the temperature at which the transition is finished. Accordingly, As and Af are the temperatures at which the reverse transformation from that repeated use of the shape memory effect may lead to a shift of the characteristic transformation temperatures (this effect is known as functional fatigue, as it is closely related with a change of microstructural and functional properties of the material).
The transition from the martensite phase to the austenite phase is only dependent on temperature and stress, not time, as most phase changes are, as there is no diffusion involved. Similarly, the austenite structure gets its name from steel alloys of a similar structure. It is the reversible diffusionless transition between these two phases that allow the special properties to arise. While martensite can be formed from austenite by rapidly cooling carbon-steel, this process is not reversible, so steel does not have shape memory properties.
In this figure, ξ (T) represents the martensite fraction. The difference between the heating transition and the cooling transition give rise to the shape of the curve depends on the material properties of the shape memory alloy, such as the alloying and work hardening.
One-way vs. two-way Shape Memory
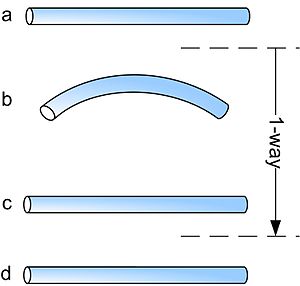
Shape memory alloys may have different kinds of shape memory effect. The two most common memory effects are the one-way and two-way shape memory. A schematic view of the two effects is given in the figure below.
In the figure above, the procedures are very similar: starting from martensite (a), adding a reversible deformation for the one-way effect or severe deformation with an irreversible amount for the two-way (b), heating the sample (c) and cooling it again (d).
One Way Memory Effect
When a shape memory alloy is in its cold state (below As), the metal can be bent or stretched into a variety of new shapes and will hold that shape until it is heated above the transition temperature. Upon heating, the shape changes back to its original shape, regardless of the shape it was when cold. When the metal cools again it will remain in the hot shape, until deformed again.
With the one-way effect, cooling from high temperatures does not cause a macroscopic shape change. A deformation is necessary to create the low-temperature shape. On heating, transformation starts at As and is completed at Af (typically 2 to 20 °C or hotter, depending on the alloy or the loading conditions). As is determined by the alloy type and composition. It can be varied between −150 °C and maximum 200 °C.
Two Way Memory Effect
The two-way shape memory effect is the effect that the material remembers two different shapes: one at low temperatures, and one at the high temperature shape.A material that shows a shape memory effect during both heating and cooling is called two-way shape memory.This can also be obtained without the application of an external force (intrinsic two-way effect). The reason the material behaves so differently in these situations lies in training. Training implies that a shape memory can "learn" to behave in a certain way. Under normal circumstances, a shape memory alloy "remembers" its high-temperature shape, but upon heating to recover the high-temperature shape, immediately "forgets" the low-temperature shape. However, it can be "trained" to "remember" to leave some reminders of the deformed low-temperature condition in the high-temperature phases. There are several ways of doing this.
If a shape memory alloy is heated up to very high temperatures (after it has been trained) then it may lose the two way memory effect, this process is known as "amnesia".
Pseudo-Elasticity
One of the commercial uses of shape memory alloy involves using the pseudo-elastic properties of the metal during the high temperature (austenitic) phase. The frames of reading glasses have been made of shape memory alloy as they can undergo large deformations in their high temperature state and then instantly revert back to their original shape when the stress is removed. This is the result of pseudo-elasticity; the martensitic phase is generated by stressing the metal in the austenitic state and this martensite phase is capable of large strains. With the removal of the load, the martensite transforms back into the austenite phase and resumes its original shape.
This allows the metal to be bent, twisted and pulled, before reforming its shape when released. This means the frames of shape memory alloy glasses are claimed to be "nearly indestrutable" because it appears no amount of bending will result in permanent plastic deformation.
History
The first reported steps towards the discovery of the shape memory effect were taken in the 1930s. According to Otsuka and Wayman (1998), A. Ölander discovered the pseudoelastic behavior of the Au-Cd alloy in 1932. Greninger & Mooradian (1938) observed the formation and disappearance of a martensitic phase by decreasing and increasing the temperature of a Cu-Zn alloy. The basic phenomenon of the memory effect governed by the thermoelastic behavior of the martensite phase was widely reported a decade later by Kurdjumov & Khandros (1949) and also by Chang & Read (1951).
The nickel-titanium alloys were first developed in 1962–1963 by the Naval Ordnance Laboratory and commercialized under the trade name Nitinol (an acronym for Nickel Titanium Naval Ordnance Laboratories). Their remarkable properties were discovered by accident. A sample that was bent out of shape many times was presented at a laboratory management meeting. One of the associate technical directors, Dr. David S. Muzzey, decided to see what would happen if the sample was subjected to heat and held his pipe lighter underneath it. To everyone's amazement the sample stretched back to its original shape.[1]
There is another type of S.M.A., called a ferromagnetic shape memory alloy (FSMA), that changes shape under strong magnetic fields. These materials are of particular interest as the magnetic response tends to be faster and more efficient than temperature-induced responses.
Metal alloys are not the only thermally-responsive materials; shape memory polymers have also been developed, and became commercially available in the late 1990s.
Crystal structures
Why does a metal or an alloy observe these qualities of memory? Dr. Frederick E. Wang,[2] an expert in crystalline structures, was the one who came with the early answers to the phenomenon. According to him, Nitinol undergoes phase changes while remaining a solid. Normally these phase changes occur in an alloy when heated to its melting point. Different phase changes occur at different temperatures.[3] These phase changes, known as martensite and austenite, "involve the rearrangement of the position of particles within the crystal structure of the solid." In shape memory alloys, these phase transformations occur below its melting point. Thus, the alloys can retain their shape without melting. Some alloys change shape within a small difference in temperature. Under the transition temperature, Nitinol is in the martensite phase and can be bent into various shapes. To set the "parent shape" the metal must be held in position and heated to about 500 °C. (varies for different SMAs). The high temperature causes the atoms to arrange themselves into a high symmetry, often cubic arrangement known as the austenite phase. In the low temperature martensite each atom "remembers" which should be its neighbors in austenite, even when the metal is deformed by crystal twinning deformation.
Manufacture
Shape memory alloys are typically made by casting, using vacuum arc metling or induction melting. These are specialist techniques used to keep impurities in the alloy to a minimum and ensure the metals are well mixed. The ingot is then hot rolled into longer sections and then drawn to turn it into wire.
The way in which the alloys are "trained" depends on the properties wanted. The "training" dictates the shape that the alloy will remember when it is heated. This occours by heating the alloy so that the dislocations re-order into stable positions, but not so hot that the material recrystallises. They are heated to between 400 °C and 500 °C for 30 minutes. Typical variables for some alloys are 500 °C and for more than 5 minutes.
They are then shaped while hot and are cooled rapidly by quenching in water or by cooling with air.
Properties
The copper-based and NiTi (nickel and titanium)-based shape memory alloys are considered to be engineering materials. These compositions can be manufactured to almost any shape and size.
The yield strength of shape memory alloys is lower than that of conventional steel, but some compositions have a higher yield strength than plastic or aluminium. The yield stress for NiTi can reach 500 [MPa]. The high cost of the metal itself and the processing requirements make it difficult and expensive to implement SMAs into a design. As a result, these materials are used in applications where the superelastic properties or the shape memory effect can be exploited. The most common application is in actuation.
One of the advantages to using shape memory alloys is the high level of recoverable plastic strain that can be induced. The maximum recoverable strain these materials can hold without permanent damage is up to 8% for some alloys. This compares with a maximum strain 0.5% for conventional steels.
Applications
The first consumer commercial application for the material was as a shape memory coupling for piping, e.g. oil line pipes for industrial applications, water pipes and similar types of piping for consumer/commercial applications. The late 1980s saw the commercial introduction of Nitinol as an enabling technology in a number of mininally invasive endovascular medical applications. While more costly than stainless steel, the self expanding properties of Nitinol alloys manufactured to BTR (Body Temperature Response), have provided an attractive alternative to balloon expandable devices. On average, 50% of all peripheral vascular stents currently available on the worldwide market are manufactured with Nitinol.
The range of applications for SMAs has been increasing in recent years, with one major area of expansion being medicine: for example, the development of dental braces that exert a constant pressure on the teeth. Patented by George Andreasen [4] who changed the formula and then formally introduced the use of Nitinol on July 26, 1979, U.S. Pat. No. 4,037,324 an American orthodontist and inventor in 1972, for use in arch wires to straighten teeth it revolutionized orthodontal medicine as well as fiber optic development because it returns to its original shape after being bent. The alloy has a patterned shape memory which expands and contracts to given temperatures because of its geometric programming.
Harmeet D. Walia later utilized the alloy in the manufacture of root canal files in endodontics. There have also been limited studies on using these materials in robotics (such as "Roboterfrau Lara" [1]), as they make it possible to create very light robots. Weak points of the technology are energy inefficiency, slow response times, and large hysteresis.
Eyeglass frames made from titanium-containing SMAs are marketed under the trademarks Flexon and TITANflex.
Nitinol wire is also used in robotics (e.g. the hobbyist robot Stiquito) and in a few magic tricks, particularly those involving heat and shapeshifting.
Boeing, General Electric Aircraft Engines, Goodrich Corporation, NASA, and All Nippon Airways developed the Variable Geometry Chevron using shape memory alloy that reduces aircraft's engine noise. Boeing's upcoming aircraft, the 787 and the 747-8 will be equipped with this new technology. [2]
Materials
Materials having the memory effect at different temperatures and at different percentages of its solid solution contents.
- Ag-Cd 44/49 at.% Cd
- Au-Cd 46.5/50 at.% Cd
- Cu-Al-Ni 14/14.5 wt.% Al and 3/4.5 wt.% Ni
- Cu-Sn approx. 15 at.% Sn
- Cu-Zn 38.5/41.5 wt.% Zn
- Cu-Zn-X (X = Si, Al, Sn)
- Fe-Pt approx. 25 at.% Pt
- Mn-Cu 5/35 at.% Cu
- Fe-Mn-Si
- Pt alloys
- Co-Ni-Al
- Co-Ni-Ga
- Ni-Fe-Ga
- Ti-Pd in various concentrations
- Ni-Ti (~55% Ni)
External links
- Memry, a large Nitinol manufacturer
- Nitinol technology (from Nitinol Devices & Components)
- Shape Memory Alloys and Their Applications - Introductory information on Shape Memory Alloys
- Nitinol Technical Data/Application Notes (from Johnson Matthey, Inc.)
- Introductions and comparisons of smart active materials (from Midé Technology Corporation)
- BBC report on medical applications of Nitinol
- SFB 459: A German Research Center for Shape Memory Alloys
- SMAterial.com - phenomena, crystallography, model, simulation and applications of SMA - Has .gif animations demonstrating the effect.
- Texas A&M University's Shape Memory Alloy Research Team - SMA overview, publications, etc.
- Institute of Physics, ASCR - Research Projects, publications, events & conferences, functional materials
- Berkeley - Bioeng/ME C117 Structural Aspects of Biomaterials - Video Lecture: Dr. Scott Robertson, Stents: Fatigue and Fracture, LBL - Material properties of Nitinol stents
- Berkeley - Bioeng/ME C117 Structural Aspects of Biomaterials - Video Lecture: Dr. Alan Pelton, Stent Design, Nitinol Device Company (NDC) - Material properties of Nitinol stents
- Thin film and Porous TiNi material source
- Nimesis - Presentation of shape memory alloys
- Endosmart GmbH, Shape Memory and Superelastic Alloys (Producer of Medical Devices)
- SMST Proceedings
- Tech Tube
- some patents and publications to memory alloys Template:De icon
References
- ^ Kauffman, George, and Isaac Mayo. "Memory Metal." Chem Matters Oct. 1993: 4-7.
- ^ Shape Memory Alloys
- ^ Binary Solid Solution
- ^ U.S. patent 5,018,969
- Duerig, TW, KN Melton, D Stöckel and CM Wayman. "Engineering Aspects of Shape Memory alloys". ISBN 0-7506-1009-3. London: Butterworth Heinemann, 1990.
- K. Shimizu and T. Tadaki, Shape Memory Alloys, H. Funakubo, Ed., Gordon and Breach Science Publishers, 1987