Sulfuric acid
| Sulfuric acid | |
|---|---|
| |
| General | |
| Systematic name | Sulfuric acid |
| Other names | Oil of Vitriol |
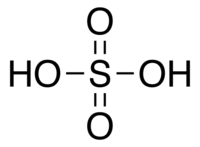
| Molecular formula | H2SO4 |
| Molar mass | 98.08 g/mol |
| Appearance | Clear, colorless, odorless oil |
| CAS number | [7664-93-9] |
| Properties | |
| Density and phase | 1.84 g/cm3, liquid |
| Solubility in water | fully miscible (exothermic!) |
| Melting point | 10 °C (283 K) |
| Boiling point | 337 °C (610 K) |
| pKa | -3.0 1.99 |
| Viscosity | 26.7 cP at 20 °C |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | very corrosive |
| NFPA 704 | |
| Flash point | not flammable |
| R/S statement | R: 35 S: 26, 30, 45 |
| RTECS number | WS5600000 |
| Supplementary data page | |
| Structure & properties | n, εr, etc. |
| Thermodynamic data | Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Related strong acids | Selenic acid hydrochloric acid nitric acid |
| Related compounds | Hydrogen sulfide Sulfurous acid Peroxymonosulfuric acid Phosphoric acid |
| Except where noted otherwise, data are given for materials in their standard state (at 25°C, 100 kPa) Infobox disclaimer and references | |
Sulfuric acid (British English: sulphuric acid), H2SO4, is a strong mineral acid. It is soluble in water at all concentrations. The old name for sulfuric acid was oil of vitriol. Sulfuric acid has many applications, and is produced in larger amounts than any other chemical besides water. World production in 2001 was 165 million tonnes, with an approximate value of $8 billion. Principal uses include fertilizer manufacturing, ore processing, chemical synthesis, wastewater processing, and oil refining.
Physical properties
Forms of sulfuric acid
Although 100% sulfuric acid can be made, this loses SO3 at the boiling point to produce 98.3% acid. The 98% grade is also more stable for storage, thus it is the usual form for "concentrated" sulfuric acid. Other concentrations of sulfuric acid are used for different purposes. Some common concentrations are:
- 33.5%, battery acid (used in lead-acid batteries)
- 62.18%, chamber or fertiliser acid
- 77.67%, tower or Glover acid
- 98%, concentrated
Different purities are also available. Technical grade H2SO4 is impure and often colored, but it is suitable for making fertiliser. Pure grades such as US Pharmacopoeia (USP) grade are used for making pharmaceuticals and dyestuffs.
When high concentrations of SO3(g) are added to sulfuric acid, H2S2O7 forms. This is called fuming sulfuric acid or oleum or, less commonly, Nordhausen acid. Concentrations of oleum are either expressed in terms of % SO3 (called % oleum) or as "% H2SO4 (the amount made if H2O were added); common concentrations are 40% oleum (109% H2SO4) and 65% oleum (114.6% H2SO4). Pure H2S2O7 is in fact a solid, melting point 36 °C.
Polarity and conductivity
Anhydrous H2SO4 is a very polar liquid, with a dielectric constant of around 100. This is due to the fact that it can dissociate by protonating itself, a process known as autoprotolysis,[1] which occurs to a high degree, more than 10 billion times the level seen in water:
- 2 H2SO4 ⇌ H3SO4+ + HSO4−
This allows protons to be highly mobile in H2SO4. It also makes sulfuric acid an excellent solvent for many reactions. In fact, the equilibrium is more complex than shown above. 100% H2SO4 contains the following species at equilibrium (figures shown as mmol per kg solvent): HSO4− (15.0), H3SO4+ (11.3), H3O+ (8.0), HS2O7− (4.4), H2S2O7 (3.6), H2O (0.1).
Chemical properties
Reaction with water
The hydration reaction of sulfuric acid is highly exothermic. If water is added to concentrated sulfuric acid, it can boil and spit dangerously. One should always add the acid to the water rather than the water to the acid. This can be remembered through mnemonics such as "Do as you oughta: add acid to water" or "Drop acid, not water." Note that part of this problem is due to the relative densities of the two liquids. Water is less dense than sulfuric acid and will tend to float above the acid. The reaction is best thought of as forming hydronium ions, as such:
H2SO4 + H2O → H3O+ + HSO4-.
Because the hydration of sulfuric acid is thermodynamically favorable (ΔH = 880 kJ/mol), sulfuric acid is an excellent dehydrating agent, and is used to prepare many dried fruits. The affinity of sulfuric acid for water is sufficiently strong that it will take hydrogen and oxygen atoms out of other compounds; for example, mixing glucose (C6H12O6) and concentrated sulfuric acid will give elemental carbon and water which is absorbed by the sulfuric acid (which becomes slightly diluted): C6H12O6 → 6C + 6H2O.
Other reactions of sulfuric acid
As an acid, sulfuric acid reacts with most bases to give the corresponding sulfate. For example, copper(II) sulfate, the familiar blue salt of copper used for electroplating and as a fungicide, is prepared by the reaction of copper(II) oxide with sulfuric acid:
Sulfuric acid can be used to displace weaker acids from their salts, for example sodium acetate gives acetic acid:
H2SO4 + CH3COONa → NaHSO4 + CH3COOH
Likewise the reaction of sulfuric acid with potassium nitrate can be used to produce nitric acid, along with a precipitate of potassium bisulfate. With nitric acid itself, sulfuric acid acts as both an acid and a dehydrating agent, forming the nitronium ion NO2+, which is important in nitration reactions involving electrophilic aromatic substitution. This type of reaction where protonation occurs on an oxygen atom, is important in many reactions in organic chemistry, such as Fischer esterification and dehydration of alcohols.
Sulfuric acid reacts with most metals in a single displacement reaction to produce hydrogen gas and the metal sulfate. Dilute H2SO4 attacks iron, aluminium, zinc, manganese and nickel, but tin and copper require hot concentrated acid. Lead and tungsten are, however, resistant to sulfuric acid. The reaction with iron (shown) is typical for most of these metals, but the reaction with tin is unusual in that it produces sulfur dioxide rather than hydrogen.
Environmental aspects
Sulfuric acid is a constituent of acid rain, being formed by atmospheric oxidation of water and sulfur dioxide. Sulfur dioxide is the main product when sulfur-containing fuels such as coal or oil are burned.
Sulfuric acid is a major component in the hot atmosphere of the planet Venus, and as a result exploration of Venus with spacecraft is difficult.
History of sulfuric acid
The discovery of sulfuric acid is credited to the 9th century Islamic physician and alchemist Ibn Zakariya al-Razi (Rhases), who obtained the subtance by dry distillation of minerals including iron (II) sulfate heptahydrate, FeSO4 • 7H2O, called green vitriol, and copper(II) sulfate pentahydrate, CuSO4 • 5H2O, called blue vitriol. When heated, these compounds decompose to iron(II) oxide and copper(II) oxide, respectively, giving off water and sulfur trioxide, which combine to produce a dilute solution of sulfuric acid. This method was popularized in Europe through translations of Islamic treatises and books by European alchemists, such as the 13th-century German Albertus Magnus. For this reason, sulfuric acid was known to medieval European alchemists as oil of vitriol and spirit of vitriol, among other names.
In the 17th century, the German-Dutch chemist Johann Glauber prepared sulfuric acid by burning sulfur together with saltpeter (potassium nitrate, KNO3), in the presence of steam. As the saltpeter decomposes, it oxidizes the sulfur to SO3, which combines with water to produce sulfuric acid. In 1736, Joshua Ward, a London pharmacist, used this method to begin the first large-scale production of sulfuric acid.
In 1746 in Birmingham, John Roebuck began producing sulfuric acid this way in lead-lined chambers, which were stronger, less expensive, and could be made larger than the glass containers which had been used previously. This lead chamber process allowed the effective industrialization of sulfuric acid production, and with several refinements remained the standard method of production for almost two centuries.
John Roebuck's sulfuric acid was only about 35–40% sulfuric acid. Later refinements in the lead-chamber process by the French chemist Joseph-Louis Gay-Lussac and the British chemist John Glover improved this to 78%. However, the manufacture of some dyes and other chemical processes require a more concentrated product, and throughout the 18th century, this could only be made by dry distilling minerals in a technique similar to the original alchemical processes. Pyrite (iron disulfide, FeS2) was heated in air to yield iron (II) sulfate, FeSO4, which was oxidized by further heating in air to form iron(III) sulfate, Fe2(SO4)3, which when heated to 480 °C decomposed to iron(III) oxide and sulfur trioxide, which could be passed through water to yield sulfuric acid in any concentration. The expense of this process prevented the large-scale use of concentrated sulfuric acid.
In 1831, the British vinegar merchant Peregrine Phillips patented a far more economical process for producing sulfur trioxide and concentrated sulfuric acid, now known as the contact process. Essentially all of the world's supply of sulfuric acid is now produced by this method.
Manufacture
Sulfuric acid is produced from sulfur, oxygen and water via the contact process. In the first step sulfur is burned to produce sulfur dioxide. This is oxidised to sulfur trioxide using oxygen in the presence of a vanadium(V) oxide catalyst. Finally the sulfur trioxide is treated with water (in the form of 97-98% H2SO4) to produce 98-99% sulfuric acid. Alternatively, the SO3 is absorbed into H2SO4 to produce oleum (H2S2O7), which is then diluted to form sulfuric acid.
- (1) Template:Sulfur(Solid|s) + O2(Gas|g) → SO2(g)
(2) 2 SO2 + O2(g) → SO3(g) (in presence of V2O5)
(3) SO3(g) + H2O(l) → H2SO4(l)
In 1993, US production of sulfuric acid amounted to 36.4 million tonnes. World production in 2001 was 165 million tonnes.
Uses
Sulfuric acid is a very important commodity chemical, and indeed a nation's sulfuric acid production is a good indicator of its industrial strength.[2] The major use (60% of total worldwide) for sulfuric acid is in the "wet method" for the production of phosphoric acid, used for manufacture of phosphate fertilisers as well as sodium triphosphate for detergents. In this method phosphate rock is used, and more than 100 million tonnes is processed annually. This raw material is shown below as fluorapatite, though the exact composition may vary. This is treated with 93% sulfuric acid to produce calcium sulfate, hydrogen fluoride (HF) and phosphoric acid. The HF is removed as fluorosilicic acid. The overall process can be represented as
- Ca5F(PO4)3 + 5 H2SO4 + 10 H2O → 5 CaSO4·2 H2O + HF + 3 H3PO4
Sulfate fertilisers such as ammonium sulfate are manufactured using sulfuric acid, although in smaller quantities than phosphates.
Another important use for sulfuric acid is for the manufacture of aluminium sulfate, also known as papermaker's alum. This can react with small amounts of soap on paper pulp fibres to give gelatinous aluminium carboxylates, which help to coagulate the pulp fibres into a hard paper surface. It is also used for making aluminium hydroxide, which is used at water treatment plants to filter out impurities, as well as to improve the taste of the water. Aluminium sulfate is made by reacting bauxite with sulfuric acid:
Sulfuric acid is used for a variety of other purposes in the chemical industry. For example, it is the usual acid catalyst for the conversion of cyclohexanoneoxime to caprolactam, used for making nylon. It is used for making hydrochloric acid from salt via the Mannheim process. Much H2SO4 is used in petroleum refining, for example as a catalyst for the reaction of isobutane with isobutylene to give isooctane, a compound that raises the octane rating of gasoline (petrol). Sulfuric acid is also important in the manufacture of dyestuffs.
A mixture of sulfuric acid and water is used as the electrolyte in various types of lead-acid battery where it undergoes a reversible reaction where lead and lead dioxide are converted to lead(II) sulfate. Sulfuric acid is also the principal ingredient in some drain cleaners, used to clear blockages consisting of paper, rags, and other materials not easily dissolved by caustic solutions.
Emergencies involving sulfuric acid
The boiling of sulfuric acid when water is added often causes clouds of sulfuric acid vapor to be released, this vapor being extremely hot as well as very acidic. Fires near or involving sulfuric acid are usually fought using foam or dry earth agents, to avoid the possibilty of the acid boiling. Where water must be used, the aim is to pour water on as much and as fast as possible to cool the resulting reaction.
Fire fighters wear splash suits when dealing with sulfuric acid, to protect themselves against both the vapor and any splashes or spills.
Precautions
When mixing with water, sulfuric acid should always be added to water, never the other way round. See above for more information. As a strong acid and an oxidiser, sulfuric acid should be stored away from bases and reducing agents. It is highly corrosive even when dilute, attacking many metals such as iron and aluminium.
Gloves and goggles should be worn when handling dilute H2SO4, and the concentrated acid also requires use of a face shield and PVC apron.
External links
- Sulfuric Acid MSDS
- Chemexper catalog of Chemical Suppliers
- Commercial overview of the sulfuric acid industry
References
- ^ Handbook of Chemistry and Physics, 71st edition, CRC Press, Ann Arbor, Michigan, 1990.
- ^ N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, pp 837-845, Pergamon Press, Oxford, UK, 1984. ISBN 0080220576.
- ^ Philip J. Chenier, Survey of Industrial Chemistry, pp 45-57, John Wiley & Sons, New York, 1987. ISBN 0471010774.
