Wikipedia:Reference desk/Science
| |||||||||
How to ask a question
| |||||||||
|
| ||||||||
After reading the above, you may
. Your question will be added at the bottom of the page. | |||||||||
How to answer a question
|
| ||||||||
January 1
Origin of the universe
- The question of the origin of the matter in the universe is no longer thought to be beyond the range of science ... everything can be created from nothing ... it is fair to say that the universe is the ultimate free lunch. — Alan Guth, The Inflationary Universe: The Quest for a New Theory of Cosmic Origins
is he right?, can something be created from nothing? I don't get how....--Cosmic girl 01:50, 1 January 2006 (UTC)
(I'm sorry for the boxes, I don't know why they apeared)
- I fixed that for you --Trovatore 02:08, 1 January 2006 (UTC)
thank you:)--Cosmic girl 03:03, 1 January 2006 (UTC)
- The origin of the universe is one of those things that will make your head hurt if you think about it too much. The fact is, nobody's really sure how the universe started. But they're working on figuring it out. -- Cyrius|✎ 04:20, 1 January 2006 (UTC)
I'm not sure what the author of the book you cite might refer to, but it's true that quantum mechanics allows the creation of temporary virtual particles from nothing. Some popular science theories have indeed suggested that the big bang might amount to little more than an unusually big vacuum fluctuation from which the matter and energy that makes up the universe emerged. Such an event would be incredibly unlikely, but, given limitless time, might nonetheless be expected to occur eventually. This could be taken to imply that the entire universe is virtual, composed of matter and energy that only exist temporarily. —Ilmari Karonen (talk) 15:17, 1 January 2006 (UTC)
Interesting... and so if the universe was indeed virtual (which I think it is)there would be no need for anything else than a vacum besides the virtual matter and energy?--Cosmic girl 17:45, 1 January 2006 (UTC)
- Yeah...and all this sounds exactly like (Zen) Buddhism! deeptrivia (talk) 18:01, 1 January 2006 (UTC)
- The canonical example of creating something from nothing is Pair production. Of course, that still requires energy to make it work.--Fangz 20:07, 1 January 2006 (UTC)
Leap Seconds
Was December 31's leap second added in the morning or at night? (Going on to January 1)
Well, whichever way you look at it, 12:00:01 is still night, isn't it? Atleast in the traditional Indian system morning starts at twilight. deeptrivia (talk) 02:29, 1 January 2006 (UTC)
- It was added at 7 PM Eastern time (the time zone for the Eastern United States) which was the same as 12 midnight GMT. So whatever that works out to for where you are, that's when it was added for you. Dismas|(talk) 04:05, 1 January 2006 (UTC)
- To be more specific, leap seconds are always added to the end of the old day as reckoned by UTC; as Dismas notes, the time in your local time zone will depend on where you are. Normally 23:59:59 UTC is followed by 00:00:00 of the new day (which is also 24:00:00 of the old day according to ISO 8601, so that midnight belongs to both days). With a leap second, 23:59:59 is followed by 23:59:60, then by 00:00:00 (or 24:00:00).
- If you go still finer and look at milliseconds, say, then 23:59:59.999 is followed by 23:59:60.000 to start the leap second, then 23:59:60.001, and so on up to 23:59:60.999, then finally by 00:00:00.000 (or 24:00:00.000). In Eastern Time in the US, similarly, the time 6:59:60.999 PM occurred during the leap second last night.
- --Anonymous, 2006-01-01, 20:42:24 UTC (give or take a couple of seconds)
- -it was added before going into the new year
What is the agama bean?
This paragraph is from the last page of Michael Crichton's Jurassic Park:
- Well, it was odd. They [the escaped dinosaurs] would only eat agama beans and soy, and sometimes chickens."
In the novel, the dinosaurs were engineered to suffer from lysine deficiency. Therefore they need to eat foods rich in lysine to survive in the wild. What is "agama bean"? -- Toytoy 03:57, 1 January 2006 (UTC)
- I suspect its fictional. I couldn't find any reference other than to the Crichton novels.-gadfium 04:06, 1 January 2006 (UTC)
- I also failed to find clues other than the cited paragraph in the novel. I have a copy of the "Advanced Reader's Edition" of Jurassic Park. The paragraph was not changed beween the ARE and the released final version. There are certainly some lyine-rich crops in Costa Rica. They have farmers. I just couldn't figure out why Crichton would need to fabricate the name of a bean. Maybe it was a typo. -- Toytoy 04:16, 1 January 2006 (UTC)
- According to dictionary.com and a google search, it's the name of a type of lizard, a place somewhere in the world, a company, and a few other things in languages I can't read. Nothing about a bean so far. Black Carrot 06:47, 1 January 2006 (UTC)
Evolution, absence of "intermediate forms"
Darwin stresses that the most formidable obstacle to his theory is the absence of "intermediate forms" in the geological record. His explanation is a rather weak claim that these cannot be found because the geological record is "imperfect to an extreme degree." How does modern science explain this point, which I'm sure is still brought up by creationists? --Tothebarricades 04:48, 1 January 2006 (UTC)
- There have been many discoveries since Darwin's day. See transitional fossil and List of transitional fossils.-gadfium 05:07, 1 January 2006 (UTC)
- There are lots of intermediate forms. The moths in industrial England which surived in black because they blended in with the smoke-colored trees, but were eaten when in white form. The various birds and reptiles in the Galapagos ... User:Zoe|(talk) 05:16, 1 January 2006 (UTC)
- The whale fossil record is very complete. This was not the case in Darwins day. Science is continually collecting more data. To cite Darwin and assume that there are still no intermediate fossils means they are ignorant on the topic. David D. (Talk) 05:21, 1 January 2006 (UTC)
The ignorant have been telling that lie for a century. Every time an intermediate fossil form is found, someone says, well where is the one intermediate to that! However far more important than the fossil record is the genetic record: every aspect of molecular biology has confirmed the fundamental applicability and usefulness of Darwinism as our best model for the obvious interrelateness of living things. See this week's issue of Science for a review of this year's advances: [1] alteripse 06:14, 1 January 2006 (UTC)
- Quite an easy proof for creationist to dismantle evolutionary theory would be to use DNA evidence to show that organisms are not in a nested hierarchy. We are still waiting for the damning evidence. David D. (Talk) 06:22, 1 January 2006 (UTC)
I think creationists and all those guys should like...feel guilty for what they are doing, seriously...--Cosmic girl 19:14, 3 January 2006 (UTC)
calamine formula
(no question)
A: It's . See this deeptrivia (talk) 06:21, 1 January 2006 (UTC)
Latin translation
Is there a Babel-fish style Latin translator on the internet? --hello, i'm a member | talk to me! 06:23, 1 January 2006 (UTC)
- Yes, just type into Google "free latin english translation" --
 Mac Davis ญƛ. 08:04, 1 January 2006 (UTC)
Mac Davis ญƛ. 08:04, 1 January 2006 (UTC)
who are the greatest scientist ?
(no question in body of text)
- That depends, who's opinion do you want? = Mgm|(talk) 10:29, 1 January 2006 (UTC)
- Many (but not all) of those generally regarded as the greatest scientists of the 20th century is given by the list of winners of the Nobel Prizes in the scientific divisions. However, if you're looking for an all-time honour board, Isaac Newton and Charles Darwin are two would be close to the very top of most people's. --Robert Merkel 11:12, 1 January 2006 (UTC)
- Charles Darwin? o.0 What did he do but evolution and natural selection theory? I know any physicist would rank Isaac Newton and of course Albert Einstein. --
 Mac Davis ญƛ. 11:44, 1 January 2006 (UTC)
Mac Davis ญƛ. 11:44, 1 January 2006 (UTC)
- Yeah, just evolution. Minor contribution. --Tothebarricades 17:40, 1 January 2006 (UTC)
- Oh come on, it has to be Leonardo da Vinci. He was at least 300 years ahead of his time.--Goshawk 14:08, 1 January 2006 (UTC)
- I think Leonhard Euler is probably one of them. – b_jonas 14:29, 1 January 2006 (UTC)
- Let's add Stephen Hawking to the list. -JvH 1 January 2006
- Nikola Tesla! Nobody remembers the guy, but he's pretty much the responsible for the advances that made electricity cheap, reliable and available to everyone today. So therefore, he had a huge role in the entire development of civilization in the last century! ☢ Ҡieff⌇↯ 19:59, 1 January 2006 (UTC)
- I'd say some of the more forgotten ones; Kelvin, Lavoisier and Robert Hooke made some major discoveries, yet are almost unknown compared to Newton or Einstein. smurrayinchester(User), (Talk) 20:29, 1 January 2006 (UTC)
- Nikola Tesla! Nobody remembers the guy, but he's pretty much the responsible for the advances that made electricity cheap, reliable and available to everyone today. So therefore, he had a huge role in the entire development of civilization in the last century! ☢ Ҡieff⌇↯ 19:59, 1 January 2006 (UTC)
- Let's add Stephen Hawking to the list. -JvH 1 January 2006
- I think Leonhard Euler is probably one of them. – b_jonas 14:29, 1 January 2006 (UTC)
- Charles Darwin? o.0 What did he do but evolution and natural selection theory? I know any physicist would rank Isaac Newton and of course Albert Einstein. --
- Richard Feynman —Keenan Pepper 21:14, 1 January 2006 (UTC)
- Galileo - Akamad 21:35, 1 January 2006 (UTC)
- Aristotle should get a nod. Most of his theories are wrong - and most scientists these days would call them "unscientific," not least because they're wrong - but he's hard to beat for priority and long-term influence. Personally, I second the vote for Newton. --George 21:49, 1 January 2006 (UTC)
- Hmmm. Feynman and particularly Hawking wouldn't make my list (brilliant scientists as they both were). In no particular order, Louis Pasteur, Copurnicus, and Galileo would rank well above them. I'd be hesitant about Da Vinci because he, genius that he was, he never bothered to tell the rest of the world about his discoveries.
- The other comment about "great scientists" is that, today at least, the scope for an individual scientist to make a great breakthrough has been much reduced. We've tackled much of the low-hanging fruit, and further scientific progress, particularly on the experimental side, will often be the result of a cast of thousands. --Robert Merkel 22:07, 1 January 2006 (UTC)
Copernicus hardly said anything new (see Heliocentrism) Probably won't even figure in my top 100. deeptrivia (talk) 23:01, 1 January 2006 (UTC)
- Meh, hands down. Antoine Lavoisier. Titoxd(?!? - help us) 23:58, 1 January 2006 (UTC)
- Bah! You're all wrong. Troy Hurtubise. --ParkerHiggins ( talk contribs ) 00:11, 2 January 2006 (UTC)
- If we're talking about practical uses of electricity, then Edison deserves a mention too. – b_jonas 11:13, 2 January 2006 (UTC)
- Edison wasn't a scientist. He was an engineer and entrepreneur, and especially a good manager. Read our article. --Robert Merkel 12:19, 2 January 2006 (UTC)
And then there's Tycho Brahe. although he was mostly good at collecting top scientists around him. And Mendeleyev; the period table represents quite a jump in our understanding of the world. The argument against Copernicus also goes for Darwin, I believe, because he also largely just regurgitated things that had been though of before but no-one dared say out loud. But all this and the above all focus on fairly recent (and western) history. There were quite a few impressive scientists in old Arabia and India, although I can't think of any names from the top of my head. For an extensive list of more recent names you could also look at the list of Nobel Prize winners. DirkvdM 12:40, 2 January 2006 (UTC)
- Descartes definitely belongs on any list, since his foundational work on philosophy of science was pretty important in the scientific revolution, plus he created the coordinate system that we still use today. Night Gyr 16:25, 2 January 2006 (UTC)
attitude towards religosity
(no question)
Edge detection
What type of filtering is edge detection?
- I'm not sure how to best answer your question, so I will provide you with a link to a simple edge detection scheme here instead, and from there, you can figure out what type of filtering is involved. --HappyCamper 16:21, 1 January 2006 (UTC)
Combining Impedance
When you parallel combine a capacitor with a resistor and inductor bound in series, what is the impedance? I will also need a magnitude.
Did that make sense to you? deeptrivia (talk) 17:49, 1 January 2006 (UTC)
Finding magnitude is easy. Separate real and imaginary parts, square them up, add them and take the square root. deeptrivia (talk) 17:53, 1 January 2006 (UTC)
- Yes, it did. Thank you.
Shouldn't that be ? GangofOne 10:21, 3 January 2006 (UTC)
Absolutely! Dunno how that error creeped into the LaTeX version of it. deeptrivia (talk) 20:10, 3 January 2006 (UTC)
science vs. pseudoscience
Hi, I wonder how can I, not being a scientist,distinguish real science from pseudoscience in popular science articles, regarding things like quantum mechanics, cosmology and things like the mental effect on health...because there seem to be a lot of articles that have views that sound a little far fetched to me, but they might as well be true, so I need some advice on which skeptic tools should I examine claims with, but I don't wish to dismiss them either, I just want to know if they are true or false.--Cosmic girl 18:35, 1 January 2006 (UTC)
- See pseudoscience and this excellent link on how to spot bogus science. alteripse 18:43, 1 January 2006 (UTC)
- thank you :), what about what Gerald Schroeder says, for example? --Cosmic girl 18:54, 1 January 2006 (UTC)
- From the Gerald Schroeder article and from his homepage, as far as I can tell he has not published his results in any peer reviewed journal - which is about as clear a sign of pseduoscience as there is. Raul654 19:02, 1 January 2006 (UTC)
- I had not heard of Schroeder, but there is a long tradition of pious attempts to reconcile particular readings of Genesis with particular scientific models. I think the basic hazard in such activities is that science and religion are two distinct ways of looking at the world, and they have different methods and different epistemologies (ways of deciding whether something is true). Trying to evaluate one with the methods of the other may be of interest as a mental or spiritual exercise but is unlikely to produce new knowledge because both bodies of knowledge make assumptions that cannot be proven or disproven by the methods of the other. For example, a basic assumption of science is that all natural phenomena follow regular, describable rules and meaningful assertions must at least in theory be testable against present and future knowledge and be replaceable if new evidence indicates it. In contrast, theological knowledge generally assumes the existence of God and meaningful assertions are evaluated against the fundamental premises of a specific religious perspective. This is why the whole intelligent design movement seems so dishonest to intelligent people-- it is a denial of the basic assumptions of each body of knowledge and a confusion of their methods. Schroeder seems to be reformulating some essentially religious concepts in the terms and concepts of contemporary physics. I would disagree with Raul that he is claiming to be producing new scientific knowledge and therefore I wouldn't condemn it as pseudoscience. alteripse 19:24, 1 January 2006 (UTC)
- If your unsure, try checking out the WP article on the topic. Or if that fails as well, ask here for a followup. :) TERdON 18:57, 1 January 2006 (UTC)
Thank you guys, I meant more like, is Schroeder basing his claims on coherent and testable things? or ar his asumptions about physics somewhat dubious.--Cosmic girl 22:14, 1 January 2006 (UTC)
- The article was pretty crap (only statements are those from supporters), so I edited it to add notable criticism for NPOV. From the information I can find and verify, the guy's a total crank and loon.--Fangz 01:55, 2 January 2006 (UTC
thanx, u rock, I'll take a look at the article. :) --Cosmic girl 15:58, 2 January 2006 (UTC)
Effects of H-Bombs
I read this today, at [2]
Do you remember those doctors a few years back who got together and announced that it was a simple, clear medical fact that we could not survive even a moderate attack by hydrogen bombs? They were not welcome in Washington, D.C.
Even if we fired the first salvo of hydrogen weapons and the enemy never fired back, the poisons released would probably kill the whole planet by and by.
Is there any truth to the second statement? I've never heard it before, and it doesn't show up on the Nuclear Weapon article
--JianLi 20:25, 1 January 2006 (UTC)
- Fusion bombs can't be that bad because they have actually been detonated. The first one was Ivy Mike. This description sounds more like a salted bomb. —Keenan Pepper 21:38, 1 January 2006 (UTC)
- It depends on how many bombs there are and what kinds of bombs they are — depending on the design of Teller-Ulam design weapons, they can generate a lot of fallout or they can generate very little. Whether a single nuclear attack would result in something like that described above depends very much on what assumptions you make about the number of weapons, the types of weapons, the total yields, how high above the ground they are detonated, and probably some moderate guesses about weather patterns. See our article on nuclear fallout for more information on this.
- Some people (usually those very much opposed to nuclear weapons) usually come out with calculations resembling a doomsday event, and other people (those who for various reasons think nuclear weapons are not necessarily mad) come up with more limited scenarios of the sort mocked in Dr. Strangelove: "I don't say we wouldn't get our hair mussed, but I do say no more than ten to twenty million killed, tops!...uh, depending on the breaks."
- As an aside, "those doctors" in question are probably a reference to Physicians for Social Responsibility. --Fastfission 22:06, 1 January 2006 (UTC)
- Ah, thanks. Curiously, there is no Wikipedia article on them, even though their website that you linked says that the were co-recipients of the 1985 Nobel Peace Prize, along with International Physicians for the Prevention of Nuclear War (maybe Vonnegut was referring to that group, too). The Nobel Peace Prize article, as well as the Nobel Prize official site [3] does mention the IPPNW, but not the PSR. Anyone know what's up with this? --JianLi 00:51, 3 January 2006 (UTC)
- It seems to be a member of the IPPNW. I have created a wikipedia article for them accordingly--JianLi 00:58, 3 January 2006 (UTC)
- I still don't understand why no body ever thought... where in the US the bombs would presumably be detonated, and of course their specs change things. The part where they said "poisons released would probably kill the planet by and by," I can assure you that part is completely false. No nuclear bombs release poisons. I have a suspicion that the quote you supplied is made up. Human, as a species, could survive a heavy attack by fusion bombs, but countries may not. Here is a magnificant page about effects of nuclear explosions for you, and future reference. [4] --
 Mac Davis ญƛ. 00:48, 2 January 2006 (UTC)
Mac Davis ญƛ. 00:48, 2 January 2006 (UTC)
- I suspect that the term 'poison' is being used to mean 'compination of radionucletides and toxic heavy metals' - e.g. radioactive strontium and iodine, and plutonium. Whist I have no evidence either way, it _is_ the sort of quote that would be made by someone uneducated or only passingly familar with the subject matter (so, that's journalists and politicians then). I very much doubt that they would kill the planet - but they could certianally do some dramatic localised changes Syntax 01:00, 3 January 2006 (UTC)
- You might also be interested in the concept of nuclear winter, where if enough of these weapons got off in a war, it can kick enough dust into the atmosphere to seriously interrupt delivery of sunlight to surface of the planet, resulting in very rapid climate change of the global cooling variety. User:AlMac|(talk) 11:09, 2 January 2006 (UTC)
Fonts
How do I create my own computer font? Do I need a special program (ie. one not shipped with Windows XP)? smurrayinchester(User), (Talk) 20:33, 1 January 2006 (UTC)
- Yes, I really doubt Windows ships with an outline font editor. I use Fontforge, which is ugly (at least until the new GTK interface works) but it works. It runs on Cygwin too. Hmmm, shouldn't Wikipedia have a Font editor article, or a Category:Font editors category? —Keenan Pepper 21:25, 1 January 2006 (UTC)
- You may try FontLab Studio and Fontographer. -- Toytoy 18:17, 2 January 2006 (UTC)
Chemical Properties of Mercury
I've been looking online for awhile now, and I have been unable to find; Combustibility, Acid/Base properties, and typical bond types of mercury (Hg). Any answers would be greatly appreciated
Thankyou~
Edd
- It looks like our Mercury article is missing a section on the chemistry of mercury, so I'll just tell you what I know. Mercury is not flammable. In fact, mercury(II) oxide readily decomposes into mercury metal and oxygen gas. Because it is not a very reactive metal, it only dissolves in strong acids. It forms two kinds of ions, Hg2+ and Hg22+, which is two Hg+ ions stuck together (Hg+ does not occur separately). —Keenan Pepper 01:00, 2 January 2006 (UTC)
- Elemental mercury is also highly volatile (and toxic!). Since it ionizes as a cation it can be used as a Lewis Acid in certain reactions. Being a metal, it participates in metallic bonding. -- Rune Welsh | ταλκ | Esperanza 05:20, 2 January 2006 (UTC)
Water
Hi, is it true or probable that water will run out in the world? (meaning drinkable water, not ocean water) and that wars will be fought in the future over water resources? I read that somewhere as a futurist story which was intended as a warning... do you think it is true? or is it silly? and if it is true, why aren't governments and scientific comunities more concerned?. --Cosmic girl 22:38, 1 January 2006 (UTC)
- In some places of the world, yes! There's fast rising population, deforestation, desertification, industrialization (water sucking industries), abnormalaties in rainfall, etc. Already in India, between state governments (among themselves and with neighboring countries) are tensions, disputes, and court cases over how to share river water, who gets how much, etc. The problem keeps becoming more severe year after year, and it won't be surprising if in the next 50 years, such disputes escalate into full-fledged wars. deeptrivia (talk) 22:52, 1 January 2006 (UTC)
Wow, I didn't know that! and don't we have the technology to fight that? or will we ever have it? ( like, desalinizating ocean water, or actually 'making' water like hidrogen fuel does or with nanotechnology?)--Cosmic girl 23:50, 1 January 2006 (UTC)
- These technologies can work on small scale, maybe for a million or ten million people, not for hundreds of million people. Possible solutions for a large scale problem like this include rainwater harvesting. deeptrivia (talk) 23:53, 1 January 2006 (UTC)
- Desalination technology has gotten a *lot* cheaper over the past couple of decades, to the point where wealthy countries can easily afford it for urban water supplies. Israel's Asheklon plant produces fresh water from seawater at a cost of about 50 US cents per 1000 litres [5]. So wealthy countries aren't going to have a problem. It's the poor, as usual, who will suffer.--Robert Merkel 05:40, 2 January 2006 (UTC)
- Yeah, that's quite true. However, note that the total population of Israel is less than 7 million. Some Indian cities with ~ 10 million population have desalination plants. With current technology, it would be a challenging task to desalinate water for over 1400 million people. Of course, technology is improving, so we can hope for better. The problem right now is not so much scarcity of water, but poor management and conservation. Also, 50 cents for 1000 litres looks cheap for domestic use, but it's real expensive for industrial use (think of irrigation, power plants, etc that use several mlds (million liter a day) of water in their process) deeptrivia (talk) 06:05, 2 January 2006 (UTC)
Do you drink public water? The earliest sign of water commodification occurred over the last decade when the middle and upper classes stopped insisting that everyone have access to high quality drinking water, just as they abandoned the commitment that even children of poor parents have access to good education or crime-free neighborhoods. Both the water supply and the public schools in most large American cities have dropped below the level which upper middle class people consider acceptable. Those who can afford to drink bottled water and send their children to private schools do so, just as they pay for security in their own neighborhoods. Once you no longer rely on public drinking water, public schools, public libraries, and the police, it is easy to vote for politicians who promise to cut the taxes of the rich, as long as the streets, schools, and water are "safe enough" for other people's children. Dalembert 00:09, 2 January 2006 (UTC)
thank you:) but I meant more of a worldwide seriously threatning for humanity.--Cosmic girl 00:31, 2 January 2006 (UTC)
- I offered it as another facet of the same phenomenon: water is becoming a resource to be controlled for economic gain in more and more of the world. During your lifetime it will go from being like air to being like land (i.e., bought and sold, with no one any longer having a public "right" to it), even in the US. Dalembert 00:49, 2 January 2006 (UTC)
- Yep, I can second that. It's another related global issue. Companies like Coke are facing the heat. ([6], [7]) deeptrivia (talk) 00:58, 2 January 2006 (UTC)
I really wouldn't be so worried. Global geopolitics is massively unpredictable on the 50+ years scale. 50 years ago, countries like India were only just created, and the world had no inkling of major water problems. It is entirely possible that as things get bad - and unlike, say, Global Warming, they probably can't go bad subtly and irreversibly - technological, logistical and regulatory systems will emerge to resolve it.--Fangz 02:12, 2 January 2006 (UTC)
- Highly optimistic, but probably wrong. I strongly recommend you to read this article and anything related to the subject of water wars. At the end it won't matter how much technology we may have to fix the problem if the poorest nations still can't afford to pay for it. -- Rune Welsh | ταλκ | Esperanza 05:09, 2 January 2006 (UTC)
- To add to what Dalembert said. Also in Europe, water that is purified for drinking is polluted upstream in other countries, even if both countries are part of the EU (I'm specifically thinking about Belgium, Germany and especially France polluting the water flowing into the Netherlands). If even 'civilised' and internationally organised countries can't solve this then what hope is there for poor countries, especially when there are already disputes (which is often the case - our western relative state of peace is quite unique in the history of mankind). As for if this can lead to war, I know it has in the past, but I can't remember where right now. DirkvdM 14:40, 2 January 2006 (UTC)
January 2
Peritoneum vs. Mesothelium
What's the difference between these two? The articles on them don't really make it clear. TheLimbicOne 01:54, 2 January 2006 (UTC)
Peritoneum is the abdominal space. Mesothelium is the membrane that lines it. In medical contexts the word peritoneum is used over a thousand times more often than mesothelium; in fact, peritoneal lining would be a clearer term to most doctors than mesothelium. alteripse 02:27, 2 January 2006 (UTC)
- Really? That's not how I learned it...the space is the peritoneal cavity. The peritoneum is the membrane that lines it—for instance, when performing abdominal surgery, it's the membrane that lies under the abdominal wall. Mesothelium is as I recall a more generic term for any type of epithelium that originates from mesoderm, including the peritoneum...I think. — Knowledge Seeker দ 02:54, 2 January 2006 (UTC)
- See Steman's definitions of peritoneum and mesothelium, as well as Merriam-Webster's definitions of peritoneum and mesothelium. — Knowledge Seeker দ 02:57, 2 January 2006 (UTC)
My understanding is that peritoneum is the serous membrane that lines the peritoneal cavity and consists of parietal and visceral subtypes depending on what it is attached to. Mesothelium, a part of peritoneum, is the single layer of flattened cells which overlies the areolar connective tissue base (which varies in thickness depending on its location and functional requirements). This is analogous to skin, for example, which has an epithelium covering the deeper connective tissue matrix of the dermis. Thats basically what my anatomy text says, anyhow. And it seems consistent with Stedmans definitions (see above). Mattopaedia 03:20, 2 January 2006 (UTC)
- Now that I read it, the peritoneum articles has a few glaring inaccuracies. I'll put it on my 'to do' list. Mattopaedia 03:23, 2 January 2006 (UTC)
- Damn. I have to admit to being wrong. Although in practice we non-surgeons often use the terms peritoneum and peritoneal cavity interchangeably, the precise meanings are as offered above. Sorry. alteripse 03:30, 2 January 2006 (UTC)
Thank you guys. To: mattaopedia, I'm currently working on a clean up and merge of body cavity and would LOVE to know where the inaccuracies are. TheLimbicOne 03:33, 2 January 2006 (UTC)
I just want to make sure I've got this correct. The coelom or pseudocoel (depending on the animal) develop from the mesothelium and (in the case of coelom) become the peritoneum. TheLimbicOne 03:54, 2 January 2006 (UTC)
Type IV Immunity disorder
My doctor tells me that I have Type IV immunity disorder. Due to this I get small blisters under the skin of my palm, the blisters are very itchy and spread on itching. My doctor tells me that this condition occrs when I have a prolonged infection in my body, but i feel this only happens when I am stressed. I would like more details about this disorder and how to prevent its onset. thanks
I assume he is referring to type IV hypersensitivity reaction, which we do not have an article about. Here is a brief description: [8] Good luck. alteripse 04:05, 2 January 2006 (UTC)
See also Hypersensitivity#Type IV - cell-mediated hypersensitivity --WS 20:52, 2 January 2006 (UTC)
Thanks, clearly we needed a redirect. alteripse 21:35, 2 January 2006 (UTC)
Why universe is dark?
Short answer: Because it had a beginning and is finite (see Olber's paradox). deeptrivia (talk) 04:27, 2 January 2006 (UTC)
- Last I checked, there was no evidence that the universe was finite. However, we can only see a finite distance because the universe started a finite time ago and the speed of light is finite. -- SCZenz 04:31, 2 January 2006 (UTC)
- Yeah, I meant according to the most established cosmological theory, it has to be finite, since nothing can travel faster than light. The statement can of course be rephrased as "because it had a beginning, and light has a finite speed" deeptrivia (talk) 04:37, 2 January 2006 (UTC)
- Well, the visible universe has to be finite. We don't have any way of knowing what's outside the visible universe, although I think people usually assume there's more of the same stuff. -- SCZenz 04:41, 2 January 2006 (UTC)
- Yeah, I meant according to the most established cosmological theory, it has to be finite, since nothing can travel faster than light. The statement can of course be rephrased as "because it had a beginning, and light has a finite speed" deeptrivia (talk) 04:37, 2 January 2006 (UTC)
- Doesn't a big-bang occuring a finite time ago, and the impossibility of anything (matter/waves/..) travelling faster than 300,000 m/s imply that the universe can't be any bigger than its age times the speed of light. Of course, I agree there's no way to experimentally verify the finiteness of size, and that it is expanding rapidly (at the speed of light?). deeptrivia (talk) 04:56, 2 January 2006 (UTC)
- I read in The Whole Shebang that space itself can expand faster than light (inflationary theory or something; I haven't quite read into it that much). --AySz88^-^ 05:06, 2 January 2006 (UTC)
- Okay, I'm not aware of that. Thanks! Hopefully, the original question is answered though! deeptrivia (talk) 05:37, 2 January 2006 (UTC)
- Just for the record, it's also a misconception to assume the big bang must have expanded from a single point. The universe could have been infinite from the first fractional second of its existence, and as far as I can tell from the talks I go to that's usually what people assume. It's more like the universe expanded from every point; it started infinite, and is now much bigger. (In the sense that short distances within that infinite size are now long distances within that still-infinite size.) Weird, huh? -- SCZenz 05:52, 2 January 2006 (UTC)
- Yeah, it's a challenging concept, but it makes sense once you get your head around it. Too bad journalists always write it like the big bang happened in a particular place. Probably a big source of confusion. Tzarius 08:09, 2 January 2006 (UTC)
- AND ANYWAY it's not dark, it just glows a color you can't see. See cosmic microwave background radiation. —Keenan Pepper 06:30, 2 January 2006 (UTC)
- Yup, you're right about the microwave background, but I guess in the question, "dark" was referred in the context of visibility (absence of colloquial "light"). Anyway, I guess for this question, we probably don't need to bother about whether the universe is indeed infinite, since due to the finite speed of light, such a proposition would not fit Karl Popper's criterion of falsifiability. deeptrivia (talk) 06:48, 2 January 2006 (UTC)
To ask a more elaborate question:
- If the universe is infinite then at every point in the sky there should be a star, so the entire sky should be lit up. So why isn't it?
Who was it again who is credited with coming up with this question? One answer would of course be that the assumption is wrong the universe is actually finite (as already stated). But another answer is that there is dark matter that obstructs much of the light. Wasn't there more? DirkvdM 15:20, 2 January 2006 (UTC)
- That's Olber's paradox, the first answer given. As far as physicists know Dark Matter isn't obstructing the light; we'd see evidence of that, and we don't. (It's called Dark Matter because it has no effect on light (or other particles) at all, aside from its gravitation.) -- SCZenz 16:43, 2 January 2006 (UTC)
- It's known as Olber's paradox, but the first person to phrase the question was Kepler, as the article states. GeeJo (t) (c) • 16:46, 2 January 2006 (UTC)
- That's Olber's paradox, the first answer given. As far as physicists know Dark Matter isn't obstructing the light; we'd see evidence of that, and we don't. (It's called Dark Matter because it has no effect on light (or other particles) at all, aside from its gravitation.) -- SCZenz 16:43, 2 January 2006 (UTC)
Images of the Milky Way
If the universe is finite but unbounded, there must be geodesics which loop back around to where they started. So, there should be images of the Milky Way galaxy (or even larger structures that contain it) that appear to be very far away. If we saw one of these images with a telescope, would we recognize it? Is anyone specifically looking for them? It would be a pretty big breakthrough for cosmology if they were found, right? —Keenan Pepper 07:12, 2 January 2006 (UTC)
- Er, yes, kinda. I think I read an article in NewScientist about that, how it's possible the universe is finite without end, but the study didn't find any of the predicted artifacts in the background radiation (there would supposedly be a pattern or particular wavelength as a result?). So it could still be finite but larger than the Hubble distance, and we'd never see our own galaxy anyway. Tzarius 07:59, 2 January 2006 (UTC)
hair loss
I am 25 male.I have problem of hair loss.What r the possible reasons for that and is it neccessary to oil hairs daily?Use of shampoo daily is safe or not?I am living in bombay, india.Thanx...
- See our article on baldness. It's most likely purely genetic. As the page mentions, there are now some reasonably effective medical treatments )the best known being mindoxil) that can stop further hair loss in a majority of people with genetic pattern baldness, and sometimes encourage regrowth, but they are expensive and need to be applied daily for the rest of your life or the hair will fall out. There are other options, including expensive implants or other cosmetic procedures, wearing a wig, or being proud of your bare skull. Oiling hairs and shampooing is unlikely to make much difference. --Robert Merkel 12:16, 2 January 2006 (UTC)
- My brother started losing hair at a fairly early age and was almost bold when he was in his forties (just some hair at the back and sides). I started losing hair at about the same speed at the same age, but now I'm in my forties and I can't realy be called bold. The fallout stopped when I stopeed using shampoo. Mind you, I had already switched to baby shampoo (mostly for the smell really :) ), but that didn't help. And when I stopped using that, the first few weeks it even got worse before it got better (I almost sound like a neoliberal now :) ). I do have some bold spots at the temples and the crown, but that had already set in at an early stage and hasn't gotten much worse (the fallout hasn't completely stopped, but the change was quite noticeable). However, each individual is different and this might not work for others. Also, someone once pointed out that I have split ends, but that doesn't bother me at all (why should it, anyway? Just commercial bull, I suppose). DirkvdM 15:34, 2 January 2006 (UTC)
If you value your hair more than your testicles, getting rid of them to lower your testosterone would probably stop your hair loss. Your choice of course... alteripse 17:40, 2 January 2006 (UTC)
haha, he's right, it's testosterone that causes hair loss, but it's also the cloging of the pores which don't let vitamins go to your folicles (is that spelled right) so you have to get a shampoo that has vitamins in it, and you should put some smashed birth control pills in your shampoo, because they are a good source of estrogen, and it also makes hair pretty and plants grow...not kidding, give it a try. --Cosmic girl 20:44, 2 January 2006 (UTC)
- FWIW, it's not actually testosterone but most likely 5-alpha reductase (which converts testosterone to dihydrotestosterone) that leads to baldness. --David Iberri (talk) 19:02, 5 January 2006 (UTC)
LOL, after reading the last two comments now I know why wikipedia has a disclaimer of "Wikipedia does not give medical advice". ;-) After alteripse's advice i am wondering if the user who asked about tetanus shots below is the same one that started this thread about hair loss. David D. (Talk) 20:47, 2 January 2006 (UTC)
Extreme Weather
What are the kinds of extreme weather and how does each of them form? Thank you.
- See the article Extreme weather. --Canley 11:39, 2 January 2006 (UTC)
Glycogenesis
Hi, I want to know where does glycogenesis take place? Thanks.219.65.191.160 15:55, 2 January 2006 (UTC)Dipankar Roy
- Have you read our article on gluconeogenesis? It says that most of it is in the cytoplasm, except the actions of pyruvate carboxylase at the end. Although, it could be stated clearer in the article. 217.208.26.177 16:03, 2 January 2006 (UTC)
- Or if you want glycogen synthesis, which is different from gluconeogenesis, check glycogen. alteripse 17:37, 2 January 2006 (UTC)
TWA flight 800
was there a german citizen by the name of herbst on the twa flight 800?
- See the flight manifest. Noone listed as Herbst was on the plane, but I suppose its possible (though not likely) he went under another name. GeeJo (t) (c) • 16:50, 2 January 2006 (UTC)
Rationalied Solar System
What would be the consequences of a sysytem where the the earths period (time) of orbit would be an exact multiple of its spin time and the lunar period?
- Well, for one thing it wouldnt last very long, since the spin time of the earth is increasing slightly every year (on the order of a few μs). GeeJo (t) (c) • 16:52, 2 January 2006 (UTC)
- Which would cause a change of a few seconds every million years. Whether that is much depends on the time scale the questioneer is thinking of. Also, many other things aren't constant either, such as the lunar period and Earth's orbit period. What is the reason for the question? The premise leads to an 'exact' duplication of any initial situation after one year. I suppose that is the purpose of the question, but I can't think what consequences that might have. DirkvdM 17:40, 2 January 2006 (UTC)
- It would be a very simple world indeed! Suppose the ration of orbital time period to spin time period is in the integer ratio m is to n. Then a year would consist of, the highest integer less than m/n, days. This is called the floor function, represented as . So, each year would lag behind by days. When this adds up to more than one day, we would celebrate a leap year. The process will repeat itself after every least common multiple of m and n days. Because in days, there will be an integer number of years and days.--Sayanchak 18:38, 2 January 2006 (UTC)
- I'm a bit confused. I thought he said the period of orbit was an exact multiple, in which case m/n would be an exact integer, by definition. It sounds like what you're describing is closer to the way things actually are.
- As far as I can tell, the only thing that would happen would be that the month could finally sync up with the lunar cycle (earth period-lunar period) and there would never again be a leap year(earth period-earth rotation), unless we just decided to do a few for old times' sake. I have read some interesting things about what would happen if the period of one planet was an exact multiple of the period of another planet near it. Apparently, the rhythmic pull of gravity between them would eventually send the smaller one shooting out of orbit, either inwards or outwards. Black Carrot 23:25, 3 January 2006 (UTC)
- Of course if n = 1, then . Hence no leap years will ever be needed. Resonce effects will only cone in case of a three body problem. I guess the user is just asking about one star one planet system.--Sayanchak 10:33, 4 January 2006 (UTC)
Universe
Is the universe finite or infinite? I can't seem to find the real answer...what is the scientific consensus about this?.thanx.--Cosmic girl 17:19, 2 January 2006 (UTC)
- The term "infinite" is used by cosmologists to refer to a mathematical abstraction and it is not clear how that abstract concept relates to the physical properties of the universe. see this wikipedia article --JWSchmidt 17:48, 2 January 2006 (UTC)
- The only consensus is that there isn't one. :) DirkvdM 17:56, 2 January 2006 (UTC)
hahaha!!! really? :) wow, I thought scientists knew it all! just kidding, I study psychology and I'm aware that we barely know the human psyche...but anyway, I'll keep searching, hehe. --Cosmic girl 20:45, 2 January 2006 (UTC)
water potential
I have read many articles on water potential in plant cells from many sites but can not find answer to these 2 problems: 1. what will happen if i leave a potato tissue in a sucrose solution for too long? will this damage the cell? 2. also does a potato cell have the same solute concentration as a onion cell? if not is the difference a significant one?
thank you inadvance for helping!
- The reason you cannot find the answers is because these questions appear to be addressing a specific experiment. I suspect one that you did in class. For question 1 it all depends on the concentration of the sucrose solution. You need to read up on osmosis. For question 2, consider the experiment you did with onion and potato. As you increased the solute (sugar) concentration what would happen to the cells (this is the answer to question 1)? Did it happen at the same time? If so the water potential in the two cells is the same. If not which cell changed first and what would that imply with respect to the water potentials in the respective cells? David D. (Talk) 17:38, 2 January 2006 (UTC)
indeed your assumptions are correct and i understand and value your response but i would still like to know whether there is any 'scientific theory' or evidence that states that if a cell is left in a specific solution (say 1M sucrose solution)for a extensive period of time it will be damaged, such that it will not be able to carry out diffusion to the same efficiency..on which i can base my reasonings for a proposed method in a experiment.
- A 1M solution of sugar is hypertonic with respect to a cell. Therefore, due to osmosis there will be a tendancy for the cell to shrink (plasmolysis). This means that the concentration of proteins and ions increase in the cell (less volume). At the cellular level this could cause problem for enzyme function. At the organismal level it could cause problems from a structural perspective, i.e. plants will wilt. Consider if the cell is in a hypotonic solution. Will this be more of a problem for animal cells or plant cells? Also consider how the water moves across the membrane so readily. Look up aquaporins. I hope this helps you on your way. David D. (Talk) 03:17, 7 January 2006 (UTC)
Medical: Frequency of Tetanus Shots
Read the instructions at the top. This is not a search engine. What is your question about tetanus shots? alteripse 18:18, 2 January 2006 (UTC)
See vaccination schedule. TenOfAllTrades(talk) 18:59, 2 January 2006 (UTC)
what is a ferrous non ferrous and non metallic material ?
Ever heard of google? [9] David D. (Talk) 20:42, 2 January 2006 (UTC)
- Don't bite the newbies, even if they ask us to do their homework. TenOfAllTrades(talk) 20:51, 2 January 2006 (UTC)
- You call that biting? I supplied the relevant google search links for their research. David D. (Talk) 20:57, 2 January 2006 (UTC)
- What is a ferrous non-ferrous non-metallic material? Impossible? smurrayinchester(User), (Talk) 20:54, 2 January 2006 (UTC)
- I think unobtainium might have all those properties. =P —Keenan Pepper 21:56, 2 January 2006 (UTC)
- What is a comma? Proto t c 12:43, 4 January 2006 (UTC)
Energy Conservation
If a virtual particle appears in a vacuum, hits another particle and is then destroyed, then wouldn't energy conservation be violated? Thanks 70.28.225.151 21:45, 2 January 2006 (UTC) Max
- No. —Keenan Pepper 21:55, 2 January 2006 (UTC)
- The joys of vacuum energy. See also virtual particle, of course. TenOfAllTrades(talk) 22:02, 2 January 2006 (UTC)
- If a virtual particle-antiparticle pair appears, and then one of the pair meets its corresponding antiparticle and the two mutually annhilate, then you are left with the (previously) virtual particle remaining, but a real one destroyed, and therefore there's been no net change (simplified). — Knowledge Seeker দ 22:14, 2 January 2006 (UTC)
- That's not how it happens. The virtual particle AND its anti-particle appears in vacuum, and they both annihilate instantly, so nothing is violated. See virtual particle andvacuum energy ☢ Ҡieff⌇↯ 22:13, 2 January 2006 (UTC)
- As far as I remember, such virtual particle/antiparticle pair creation in empty space is made possible by borrowing energy from the uncertainty principle. This can happen only for a duration as small as the Planck time, and according to Quantum mechanics, energy conservation is not valid for such small time durations (there are fluctuations of energy at that time scale.) This is what I read somewhere. Am I correct? deeptrivia (talk) 05:04, 3 January 2006 (UTC)
- Well, that's not really correct. In the early days of QED, people did their Feynman diagrams in a noncovariant way, so that 3-momentum was conserved, but energy was not. In those days, people liked to say that virtual particles borrowed energy from the vacuum. These days people do covariant Feynman diagrams where 4-momentum is conserved at every vertex, and they say that virtual particles live off their mass shell, which means they violate the relation p^2 = m^2 (the solution of which is a 3 dimensional hyperboloid shell in the 4 dimensional momentum space). So the answer to your question about borrowing, deeptrivia, is that it's a matter of convention, or terminology. Under today's conventions, the answer is no, no such violation occurs. The reason that a seemingly well-defined question "do virtual particles violate conservation of energy?" can fail to have a unique answer is that virtual particles are just incomplete parts of a whole theory. They're not measurable. They're just terms in a series expansion, only the infinite sum of which has physical meaning which is independent of your point of view and can be measured. And of course in the physical sum, there is no violation of conservation of energy. -lethe talk 23:46, 4 January 2006 (UTC)
- As far as I remember, such virtual particle/antiparticle pair creation in empty space is made possible by borrowing energy from the uncertainty principle. This can happen only for a duration as small as the Planck time, and according to Quantum mechanics, energy conservation is not valid for such small time durations (there are fluctuations of energy at that time scale.) This is what I read somewhere. Am I correct? deeptrivia (talk) 05:04, 3 January 2006 (UTC)
Yes but if some of the energy were transfered, it could not be payed back. Max 216.209.153.123 13:26, 3 January 2006 (UTC)
- You are correct, that if such an interaction could happen, it would be a violation of conservation of energy. That's why it cannot happen. The Feynman diagram describing such a process is called a tadpole diagram, and tadpole diagrams always cancel each other out in an anomoly free field theory. I suppose there might be a more physical intuitive way to explain why this doesn't occur, but I don't know it. You might try asking at the tadpole page. -lethe talk 23:46, 4 January 2006 (UTC)
What makes tadpoles always cancel out? What does the circle represent? 216.209.153.142 13:18, 5 January 2006 (UTC) Max
- The circle represents a particle antiparticle pair. Imagine that time is the vertical axis of that picture. So at t=0, you start at the bottom, and you have one line with an arrow coming out of the bottom and pointing up the left side, and another line with an arrow going down, coming into the bottom. The second line is pointing backwards in time, since time increases as you go up, but the arrow is pointing down. An arrow that's going backwards in time represents an antiparticle, while an arrow that faces forwards in time represents a particle. Thus I have a particle going up the left, and an antiparticle going up the right. In short, there is a particle/antiparticle pair production happening at the bottom of the graph. Virtual particles that come out of the vacuum always come in pairs like this.
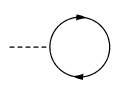
- Somewhere in the middle, the particle on the left ejects a photon (this could be a collision with a photon instead. doesn't much matter whether it's an emission or a collision, both have the issue with conservation of energy that you mentioned). as time progresses, you get to the top of the diagram, and the particle and antiparticle meet up again, and annihilate. The photon has carried off some energy, so it would appear that energy conservation has been violated.
- Now as for why these diagrams give vanishing contribution, well, for QED (photons and electrons), there's a theorem that says that any diagram with an odd number of photons interacting always gives zero. That's known as Furry's theorem. All I can say about that is, it's part of the theory. The theory is built from the ground up to respect conservation of energy, so of course in the end, conservation of energy always holds. Just do the calculation and you can verify it. I suppose this isn't a very satisfying answer. What you'd really like is for some physical description of what keeps these particles from acting in this way. Like I said before, I don't know a nice physical explanation. The best I can say is that virtual particles aren't free to do what they want the way that real particles are. Virtual particles aren't real; meaning that they're artifacts of the mathematics that we use to approximate real particles. They're only intermediate terms in an infinite sum which represents the real particles. They have to take into account all the interactions in the diagram, they can't just do what they want at each moment, and wait until later to find out if energy gets conserved. They're bookkeeping devices to aid in the calculation, and are subject to the rules of the caclulation. In QED, you can argue for the vanishing of this diagram through charge conservation or Lorentz invariance, but those won't apply to scalar QFT (like, say the theory of pions). In that case, you probably just have to plug through the calculation.
- But let me just say again, just because I don't know a physical explanation for why this interaction is ruled out, doesn't mean that there isn't one. Some really smart people can sit down and think hard about virtual particles, and give somewhat useful descriptions of how they move and how they're allowed to act. So you might try asking around. I was thinking maybe we could ask Lubos, who wrote the article tadpole. If you'd like to carry this conversation further, you might try coming to my talk page, since I don't check this page so often. -lethe talk 02:26, 6 January 2006 (UTC)
January 3
pop?
How many licks does it take to get to the center of a tootsie roll pop?--172.172.212.217 02:23, 3 January 2006 (UTC)
Science can't answer that without enough data, which I don't think you posses either.lol --Cosmic girl 02:30, 3 January 2006 (UTC)
- When I was in elementary school, I tried this little experiment. I had a brown Tootsie Pop which I licked in two ways: 1) a conventional sweep of one side of the pop against the extended tongue, 2) placing the whole pop in my mouth, closing the mouth, and pulling the pop out (for a sort of all-over "lick"). I checked the pop after each "lick" for visible Tootsie roll and, much to my surprise, saw it first at precisely 1500 "licks." Not the most valid experiment, but it's an answer. --George 03:00, 3 January 2006 (UTC)
- From the article, Tootsie Pop:
- According to the official Tootsie Roll website, Tootsie Roll Industries has received over 20,000 letters from children claiming to have solved the riddle since the commercial first aired in 1970. The typical range of responses is between 100 and 5,800 with an average of 600-800. There is no official number, as everyone's saliva and licking method is different.
- According to Tootsie Roll Industries, there have been several scientific or pseudo-scientific studies attempting to answer the "How Many Licks?" question, including the creation and testing of two unique "licking machines" by engineering students at Purdue University and the University of Michigan. —Keenan Pepper 03:10, 3 January 2006 (UTC)
- Hmm...a very interesting question. I may save it for use when I interview people for permanent job positions. --HappyCamper 03:16, 3 January 2006 (UTC)
- What for? Black Carrot 23:38, 3 January 2006 (UTC)
Non zero value
what is 'non zero value'? ( concerning the higgs boson)--Cosmic girl 03:20, 3 January 2006 (UTC)
- Oy vey. The Higgs boson is a quantum (i.e. a little piece) of the Higgs field, which is represented mathematically as a complex function (that is to say, a function whose values are complex numbers) of space and time. The Higgs potential is the energy of the field as a function of the value of the field. The reason particles have mass, according to the usual Higgs mechanism, is that the minimum value of the Higgs potential (where the universe "settles") is at a place where the complex value of the higgs field is not equal to zero. As a result, which one obtains through some calculations, certainl particles gain mass, where they were massless before. That probably doesn't fully help, so please ask more questions! -- SCZenz 04:13, 3 January 2006 (UTC)
thank you! :D u r very nice, well I know nothing about physics(I study psychology which I guess has nothing to do with physics), but I kind of understood what you said... so, now I want to know what would happen if the minimum value equaled zero? that means particles would have no mass? and if there was no mass what would there be, just nothing? because I don't understand what is mass either...haha. thanks again --Cosmic girl 19:03, 3 January 2006 (UTC)
- No mass does not necessarily mean nothing. The commonest exampe would be a photon. They have zero rest mass, yet they exist and are entering your eyes as you read these lines.--Sayanchak 10:52, 4 January 2006 (UTC)
cool :) , so what is more fundamental, a photon? or a higgs boson? (maybe I ask silly questions, but I'm not a physcist) --Cosmic girl 23:08, 4 January 2006 (UTC)
- To me that's comparing apples and oranges. The photon is the force-carrying partice for electromagnetism, one of the four fundamental forces (in fact, along with gravity, one of the two long-range forces), but a Higgs boson would be the source of all mass. Which is more fundamental, mass or force? — Laura Scudder ☎ 23:22, 4 January 2006 (UTC)
I don't know... which is? --Cosmic girl 23:38, 4 January 2006 (UTC)
Grapefruit Juice + Baking Soda Specific Heats
Does anyone know the specific heat of baking soda and grapefruit juice? Please let me know if You all find out... Thank you very much! :) Gene Sparks
- Sorry no body answered your question. Because you can't find this on the internet (you may but its hard!), you'll have to test them yourself.
- Baking Soda: Use the guide at [10] to find baking soda's specific heat. If you have any questions leave me a comment on my User_talk:Mac_Davis.
- Grapefruit Juice: Do the same thing, but obviously replacing the water with grapefruit juice, and not including the test tube and metal.
- Here's a sample equation:
- Calculate the specific heat capacity of copper given that 204.75 J of energy raises the temperature of 15g :of copper from 25˚C to 60˚C.
- q = m x Cg x (Tf - Ti)
- q = 204.75 J
- m = 15g
- Ti = 25˚C
- Tf = 60˚C
- 204.75 = 15 x Cg x (60 - 25)
- 204.75 = 15 x Cg x 35
- 204.75 = 525 x Cg
- Cg = 204.75 ÷ 204.75 = 0.39 J˚C^-1 g^-1
- Sorry, I can't do LaTeX.
- Here's a short guide for the military [11], and Britannica's [12]. Happy New Year --
 Mac Davis ญƛ. 05:32, 3 January 2006 (UTC)
Mac Davis ญƛ. 05:32, 3 January 2006 (UTC)
Physics of hitting a wall
Take a standard, solid wall. If tap on that wall with my hand, will the wall itself be moved by that? I know that common sense would say 'of course not, that's not strong enough'. But even on an INCREDIBLY small scale, did I move that wall AT ALL? Flea110 03:47, 3 January 2006 (UTC)
- Yes, because the wall is not totally incompressible. Also, some sound waves would have propagated through the wall, and that is motion. Tzarius 04:09, 3 January 2006 (UTC)
- Is the wall's physical position different 5 minutes after I hit it compared to before I hit it (as a result of me hitting it)? Flea110 04:26, 3 January 2006 (UTC)
- Almost certainly not, but it's warmer. -- SCZenz 04:28, 3 January 2006 (UTC)
- Is the wall's physical position different 5 minutes after I hit it compared to before I hit it (as a result of me hitting it)? Flea110 04:26, 3 January 2006 (UTC)
- From a "pure science" point of view, it would be impossible to say it did not move/deform, even after 5 minutes. There would be some residual plastic deformation. But in even the most accurate of engineering models, you will assume that there is no movement/deformation at all. deeptrivia (talk) 04:57, 3 January 2006 (UTC)
- To complete the last answer, a deformation can be elastic, meaning it bounces back to its original state, or plastic, meaning it stays deformed. Or a combination of the two. You'll get some elastic deformation (if not, how could anyone at the other side of the wall hear you banging?). As for plastic deformation, that can be local (a dent) or the whole wall may move. I assume your hand is too soft too make a dent in a brick wall. But whether the wall as a whole can move is more a matter of how well it is grounded. And that movement will most likely be a tilt, not a shift. So there are many variations of what you may be asking. And it's also a matter of precision. What defines the wall? Where are its boundaries? You can only say that with a certain precision. So the wall as a whole may have moved, but the movement will probably have been much smaller than that precision, so you can't say if it moved. Then again, I've seen some walls.... :) DirkvdM 12:56, 3 January 2006 (UTC)
- Put an extremely sensitive accelerometer on the wall before you lean on it. You will see that it moves, and generates vibration (seismic) waves. But the waves decay back to noise in a short time, since you will see that the wall is always shaking. The energy that you imparted to the wall propagates into the rest of the structure, and becomes heat. You will not have affected the molecular structure of the wall in any way, since all of the motion was elastic, and the amplitude was well below the normal live loads (like somebody walking by). Do the same experiment with a big earthquake, and the results are different. --Zeizmic 15:15, 3 January 2006 (UTC)
Dental Decay
what is the cuase of dental decay?
- Your mouth contains a wide variety of bacteria, but only a few specific strains of bacteria cause dental caries, mainly S. Mutans and Lactobacilli. These bacteria convert available carbohydrates into acids such as lactic acid created through fermentation processes. These acids seep into the tooth and can wear away tooth structure. If conditions in the mouth are favorable, S. Mutans and Lactobacilli will continue to thrive and continue to secrete these acids. Bacteria, acid, food debris, and saliva combine in the mouth to form a sticky substance called dental plaque that adheres to the teeth.
- It is most prominent on the grooved chewing surfaces of back molars, just above the gum line on all teeth, and at the edges of fillings. Plaque that is not removed from the teeth mineralizes into calculus (dental) (tartar). Plaque and calculus irritate the gums, resulting in gingivitis and ultimately periodontitis.
- The acids secreted by S. Mutans and Lactobacilli in the plaque dissolve the enamel surface of the tooth. As the bacteria become more prolific, the bacteria will follow the advancing front of acid damage and infect the dentin within the tooth. Left untreated, carious lesions will increase in severity from small discolored stains to actual holes in the tooth (cavities). Cavities are usually painless until they grow very large inside the internal structures of the tooth (the dentin and the pulp at the core) and can cause death of the nerve and blood vessels in the tooth. If left untreated, complications may occur such as acute pulpitis (infection of the pulp) or abcesses within the jaw.
- Plaque and bacteria begin to accumulate within 20 minutes after eating, the time when most bacterial activity occurs. If plaque and bacteria are left on the teeth, cavities can develop, and untreated tooth decay can result in death of the internal structures of the tooth and ultimately the loss of the tooth.
Dietary sugars and starches increase the risk of tooth decay. The type of carbohydrate and the timing and frequency of ingestion are more important than the amount. Sticky foods are more harmful than nonsticky foods because they remain on the surface of the teeth. Frequent snacking increases the time that acids are in contact with the surface of the tooth. Yay that's all.-- ![]() Mac Davis ญƛ. 05:16, 3 January 2006 (UTC)
Mac Davis ญƛ. 05:16, 3 January 2006 (UTC)
- Wasn't there some research done on a modified bacterium that was intended to displace s. mutans and lactobacilli in the mouth, without producing acids? IIRC they've done successful test trials on humans but are now held up at the FDA / approval stage. Can't remember any names. It'd be lovely to never have to brush again, yet always have sparkling white teeth... Tzarius 07:15, 3 January 2006 (UTC)
- Also, in Scientific American about nitrate, a preservative in processed meats, and found naturally in green vegetables. It was once considered unhealthy, and linked to link to stomach cancer. In the 1950s, researchers found that a class of these chemicals, N-nitrosamines, damages DNA, and causes cancer in rats and tested farm animals. Though, in 1994, Jon Lundberg of the Karolinska Institute in Stockholm, and Nigel Benjamin of Peninsula Medical School in Exter, England, researched and put out a report that the human stomach naturally harbors large amounts of these chemicals. Humans seem to’ve structured a symbiotic relationship with bacteria in the back of the mouth convert nitrate from food, to nitrite, which is swallowed, and chemicals in the stomach mix to create nitric oxide. The stomach is the main line of defense against ingested pathogens, though E. coli, and Salmonella can survive in it for hours, researchers found that nitric oxide killed the bacteria in minutes. Nitrites also, when ingested, cause the stomach to thicken their mucus lining, and to increase blood flow to the stomach, preventing infection and ulcers. Benjamin also found that major cavity-causing bacteria, self-destructed, when placed in a high nitrite environment, which in the future could have applications in the dental industry, and many other fields.
To return to the original poster's question, the only unequivocal facts about dental caries (tooth decay) is that three things must be present for it to occur:
- a tooth
- bacteria
- fermentable substrate
That a tooth is necessary is self-evident. The other two factors have been demonstrated experimentally by using strains of germ-free rats, which do not experience dental caries until they are innoculated with cariogenic (decay-causing) bacteria and fed carbohydrate by mouth.
This does not begin to explain the wide variability of caries experience among individuals. There are more than a few cases where the bacterial load in a given mouth is off the charts due to poor oral hygiene and poor diet, yet tooth decay is absent. The converse is also true: excellent oral hygiene and attention to diet does not assure that tooth decay will not occur or even that it will not be rampant. This suggests that there are other factors that influence the incidence and severity of tooth decay-- whether it is some intrinsic quality in a particular person's tooth enamel, salivary antibodies, salivary enzymes, or some environmental element-- be it dietary or otherwise.--Mark Bornfeld DDS 17:25, 3 January 2006 (UTC)
death star
Is the concept of death star is feasible?
- No. Currently, and in the future, no. Its too expensive. Not just money-wise, but its to big, its too bulky, it's made almost entirely of metal, maintenance costs, its a gas guzzler, and what would we use it for? You may be interested in Dyson spheres. Leave a message on my talk page if you want to know the myriad of reasons why it is either impossible or incredibly infeasible. --
 Mac Davis ญƛ. 05:40, 3 January 2006 (UTC)
Mac Davis ญƛ. 05:40, 3 January 2006 (UTC)
- Also see this guy's technical analysis of the Death Star. The quantities of energy required to destroy planets in the manner depicted in the movie is so enormous as to be highly unlikely, no matter how much technology improves. Not to mention wasteful; why bother vaporizing a planet when all you really need to do is wipe out the biosphere? --Robert Merkel 06:30, 3 January 2006 (UTC)
- Suppose a way could be found to make the hydrogen in the oceans undergo nuclear fusion, would that blow up a planet?
- Why bother? Because it looks really cool! :-) Dismas|(talk) 06:43, 3 January 2006 (UTC)
- Also see this guy's technical analysis of the Death Star. The quantities of energy required to destroy planets in the manner depicted in the movie is so enormous as to be highly unlikely, no matter how much technology improves. Not to mention wasteful; why bother vaporizing a planet when all you really need to do is wipe out the biosphere? --Robert Merkel 06:30, 3 January 2006 (UTC)
- Indeed, this page shows a rough calculation of how much energy is needed to disperse an earth-sized planet, which works out to be approximately 2.4e32 joules (or 57,361,376,673,040,140 megatons of TNT). In terms of matter / antimatter destruction, you'd need about 2,670,360,134,547,751kg of matter and antimatter, which in a ball at the same density as Earth, would be about 9,742km across. Pretty large, if you ask me. (PS: that assumes 100% energy->KE conversion efficiency) Tzarius 10:39, 3 January 2006 (UTC)
- Here are a couple statements from the Star Destoyer link.
Statement #1
"Alderaan might have been a very tiny planet, so it would have been very easy to destroy". There is a fairly narrow range of planet types which can support human life. A human-habitable planet must have sufficient gravity to have retained an atmosphere after its formation, so Alderaan simply could not have been an extremely small planet. Furthermore, the Death Star has been explicitly described to be capable of destroying any inhabited planet.
OK, but Alderan may have been a small, ARTIFICALLY created planet. So it could have been build using superdense material material at the core to give it enhanced gravity.
Statement #2
So if we can't use melting energy or vaporization energy, how do we determine the energy requirement to destroy a planet? The answer, in one word, is gravity. If you wish to destroy a planet, you must scatter its mass so quickly that the forces of gravity cannot reverse the expansion process. In other words, you must accelerate the planet's entire mass to escape velocity. Another way of saying this is that you must bring the planet's gravitational potential energy state up to zero. The concepts of gravititational potential energy and escape velocity are both discussed in the science page. Using those concepts, the energy requirement for blasting a planet apart can be calculated.
The science page alludes to, but I cannot find where it says explicitly, that objects at the center have lower escape velocity than those at the surface. This is because the outer surface of the planet has gravity and tends to attract that at the center, away from the center. So it takes less energy to drive away the material at the center, than at the surface.
Depends, do you mean using current known physics, or unknown physics? Is FTL travel possible? What about time travel? I think that yes, a Death Star is feasible, but not with current technology, as with many other things.
If you really want to kill everyone on a planet, a Death Star is an overblown (sic) and hideously bombastic way of doing so. It's far easier, and altogether more useful, to do what the aliens do in the novel Footfall. You just take your moderate sized spaceship out to the asteroid belt, grab a nice sized asteroid, and drag it back toward your target planet, engines at full blast all the way in. Turn aside at the last minute (or, better, use a robot spaceship for the whole deal) and let the asteroid whack into the target planet. The poor inhabitants suffer a giant tsunami and an impact winter. You can come back in a decade or so and find a nice uninhabited planet, with the climate returning to normal and all the life-sustaining lower organisms intact, but all the people who defied you killed in way the average interstellar-villain type will find particularly gratifying. -- Finlay McWalter | Talk 20:01, 3 January 2006 (UTC)
- Or better yet, why not use a virus that mutates to infect and destroy all life on the planet ? Such a weapon was contemplated in a Star Trek: The Next Generation episode. StuRat 22:35, 3 January 2006 (UTC)
- Nah, viruses and other too-clever-by-half stuff are for amateur-hour villains. Sure, your minions told you that by the time you landed a couple of years later the virus would be gone, but you just know it'll be hiding in some lichen or something, just waiting for you to make your triumphal tour of inspection. The nice thing about the asteriod is the guarantee of no nasty surprises later - one big enough to kill all large animals is way too small to cause wholesale tectonic problems. You just need to remember to survey the impressive crater from orbit, and not by walking around on its edge saying prideful things. -- Finlay McWalter | Talk 23:26, 3 January 2006 (UTC)
jackal
What are the good qualities of jackal which are used as to describe to a wise, clever person?
- Tale of Two Cities I presume? :)
- Dickens devoted Chapter 5, Book the Second to Sydney Carton, whom he nicknames “the jackal.” A jackal can also mean accomplice in the commission of menial or disreputable acts—the name seems fitting, for his situation. --
 Mac Davis ญƛ. 09:32, 3 January 2006 (UTC)
Mac Davis ญƛ. 09:32, 3 January 2006 (UTC)
- Oh, sorry I may not have answered your question, erm.. jackals seize the opportunity to kill when they can. That may be smart. They work in twos, you could say, even though its just monogamous heterosexual relationships. A jackal may remind you of the cunning fox. --
 Mac Davis ญƛ. 09:46, 3 January 2006 (UTC)
Mac Davis ญƛ. 09:46, 3 January 2006 (UTC)
- Oh, sorry I may not have answered your question, erm.. jackals seize the opportunity to kill when they can. That may be smart. They work in twos, you could say, even though its just monogamous heterosexual relationships. A jackal may remind you of the cunning fox. --
Methane leakage from barometric pressure
Ok, this question might lead to a long answer, but here goes: I was reading this article [13] about the recently trapped coal miners and I came across this tidbit:
- Coal mine explosions are typically caused by buildups of naturally occurring methane gas, and the danger increases in the winter months, when the barometric pressure can release the odorless, colorless and highly flammable gas.
How does this work? Is the winter average atmospheric pressure normally higher or lower than the summer (where I live I would guess lower because its raining all the time, but in places with cold clear winters wouldn't the pressure be higher?) How would a higher pressure cause the release of more methane than usual? If it's a lower pressure, am I correct in assuming that it has to do with the pressure relative to the vapour pressure?. And btw, Coal mining doesn't contain the answers (yet) so don't bother looking. -User:Lommer | talk 07:47, 3 January 2006 (UTC)
- Maybe the methane was under pressure, in its own pocket inside surrounding earth. --
 Mac Davis ญƛ. 09:34, 3 January 2006 (UTC)
Mac Davis ญƛ. 09:34, 3 January 2006 (UTC)
- Maybe the methane was under pressure, in its own pocket inside surrounding earth. --
- Take a look at Wind Cave National Park. I am sure that, away from the coast, the biggest deltas (air pressure) would come from winter storms. --Zeizmic 12:57, 3 January 2006 (UTC) p.s. this is a good read. [[14]]
Architecture
1. what are the guidelines for gesigning galery spaces?
2. what are the best conditions for showcasing art works?
3. how are the techniques of lighting employed in the lighting of artifacts?
4. what are current trends in the design of art exhibition facilities?
I'll be most grateful to have these information. Thank you.--81.199.78.102 17:25, 3 January 2006 (UTC)
- Could you let us know something about why you want this information? The reason for asking is that they do look a bit like homework questions. Numbering the parts of the question makes it look especially that way. Notinasnaid 17:29, 3 January 2006 (UTC)
I am an architecture student tasked to design a "National Museum" and its poving dificult getting these information due lack of an appopriate case study in my country Ghana. hank you.--81.199.78.102 00:10, 4 January 2006 (UTC)
- I won't discuss the obvious things, like having a comfortable environment for the viewers of the art work, but will mention some more obscure considerations:
- Large freight elevators must be available for lifting heavy objects, like massive sculptures, to the desired floors. Halls must be wide enough for fork lifts capable of carrying such works, as well.
- In addition to the exhibit space, large amounts of warehouse space are needed to store objects not currently on display. An administration/personnel area is also needed. Gift shops, rest rooms, and cafeterias for the visitors may also be desired. The gift shops and cafeterias could be a source of financing for the museum, as well.
- Light, especially sunlight, can damage many artifacts. The Shroud of Turin, for example, is quite photo-sensitve. For this reason, the exhibit areas usually don't have windows, but rather use adjustable lighting which can be turned down for sensitive exhibits. Tract lighting, for example, allows lights to be aimed at selected works, and away from others.
- Security is a serious concern, since artwork may be worth millions. Placing portable, valuable art work near an emergency exit, for example, is a mistake, as a thief could smash the protective glass and remove the item then head out of the exit.
- Fire suppresion is problematic in musuems, as water sprinklers used to control fire elsewhere can cause serious damage to art work. Use of nonflammable construction materials, like reinforced concrete, instead of wood, is critical. Areas with artwork which can be damaged by water should have such works covered in airtight containers and possibly also use a non-water based extinguishing system, such as Halon.
- The flow of visitors through the museum should also be considered. It is more efficient if visitors can see everything without going through the same room twice. For example, a central atrium with numerous exhibition hall "loops" would keep visitors from going through any exhibit area twice, and would allow them to skip an exhibit which doesn't interest them. This will both increase satisfaction by those visitors and also will get them out of the museum as soon as possible, and thus limit overcrowding.
- Parking should be sufficent for the maximum crowd for any event, and should also have security, both to protect the public and to detect any stolen artifacts before the thief can drive off.
- A secure shipping area is needed for large sculptures which need to be handled with heavy equipment as well as small, hand-carried art objects. More mundane items, like toilet paper, could be delivered via another shipping entrance. However, beware that such an alternate shipping entrance could also be used to remove stolen artwork, so still needs substantial security, such as video cameras.
- Small children can be a problem, since they likely will get bored and cry or whine when shown abstract art which they are not allowed to touch. One possible solution is to have a "children's area" with things which they will like, and can touch without damaging, like massive marble statues of animals, for example. This becomes an architectural issue if the children's area is specially designed, with lower drinking fountains, for example, to accomodate children.
- Temperature and humidity must be rigidly controlled. Consideration should be given to underground exhibit space, as this makes temperature and humidity control easier, more reliable, and less expensive.
About the remote viewing thing... (and the Russian Revolutions)
Have gobvernments already give up on this? I can't understand how gobvts. of 1st world countrys could be doing things so cool like the space station, but also believe in those things like remote viewing...so if someone could clear this up for me. thanx.--Cosmic girl 17:43, 3 January 2006 (UTC)
- Hi Cosmic girl. See remote viewing. It's generally considered paranormal phenomena and not based on the scientific method in any meaningful way. --Quasipalm 19:23, 3 January 2006 (UTC)
- The thing is the gov is many different people, with many beliefs. THere is no one person who knows it all. The military/spy agencies sponsored RV experiments only because it MIGHT be true, just so they were covered if it were. They have a LOT of money to spread around, so they are constantly getting pitched by people who try to make them fearful. GangofOne 01:46, 4 January 2006 (UTC)
- What you mean is not remote viewing but remote sensing. The country/government that was most into space stations was the USSR. When that govt changed it drastically reduced its investment in space exploration and the like. Anyway, you don't need space stations (ie the presence of people in space) to do remote sensing. A satellite or even an aircraft will do. By the way, you talk about first world countries and this makes me wonder; is Russia now a first or a third world country? It used to be 'second world' because of its state socialism. What is it now? Given the way its redistribution of wealth is going, combined woth the overall wealth, it's starting to look more and more like a third world country. DirkvdM 09:44, 4 January 2006 (UTC)
I didn't say there needed to be space stations to remote sense... what I said was that I find remote viewing and remote sensing or whatever, silly, but I find the space program really cool, I never equated the space program with the remote viewing thing...they are two separate things. and I don't know what is Russia, but I'm sure it's not a 3rd world country, and I don't think it's a 2nd one either, and I also think that their abandonment of socialism was the best thing they could've done. --Cosmic girl 23:24, 4 January 2006 (UTC)
- This is getting rather off-topic (then again it's your thread), but the way they dropped state socialism (not just socialism, but let's not get into that) was the worst thing they could do. Give a people who have been brought up with the idea that property is theft capitalism and what do you get? A country full of thieves (and a bunch of people who sit in stupor, still trying to grasp how and why the government support dropped out from under them). In 1990 I thought that the speed of the change was intended to give people such a shock that they would massively start voting for the communist party. If that was the plan it failed, although the communist party did get quite a lot of votes (and as the economy deteriorates it may still happen). I agree that, although the revolution was necessary, the USSR system that came in the place of the Tzars was in the long run not a very good idea - it was like a ladder that had to be climbed and then thrown away. But not the way they did it in Russia. China, however, is soing it the right way it seems. DirkvdM 09:39, 5 January 2006 (UTC)
- Addition: The best thing that happened to Russia must have been the February Revolution because that finally got rid of the Tzars (there had been many many bloodily suppressed attempts before that, illustrating the need). What would have happened if the October Revolution had not taken place and if that would have been better is anyone's guess. But one can also say that there was an historical necessity for state socialism. The movement was very string and it had to happen somewhere. Now we know about a few bad ways to implement it so we can prevent those in the future, mainly the possibility of too much power in the hands of one person (which was the original problem), which gave Stalin the power to kill millions. Alas history is repeating itself. Again, there is too much power in the hands of one person in Russia. Willmankind ever learn? And is there a remedy? Democracy is no failsafe solution, because Putin, Hitler and Saddam Hussein all came to power in a democratic system. I don't have the answer either. DirkvdM 10:42, 5 January 2006 (UTC)
- Except maybe parliamentary democracy, because there, no single person has power over everything - even the prime minister has just one vote and the specific fields are left to the ministers. DirkvdM 07:37, 6 January 2006 (UTC)
- A quick note: First World doesn't have anything to do with the wealth of a nation. In fact, it is simply a carry-over from the Cold War. During the cold war, the US and its allies (mainly Western Europe) were called the First World. The Second World was the USSR, Eastern Europe, and China. The Third World was every other country not tied to a side of the Cold War. However, since the end of the Cold War, 1st World has been commonly used to describe a country with liberal policies and market economies, while the evolving meaning of 2nd and 3rd world countries isn't as clear. --Quasipalm 19:52, 6 January 2006 (UTC)
Medicine: what is alexitimia?
Dear friends What is alexitimia? Couldn't find it in the wiki search. Thanks in advance.
- Do you mean Alexithymia? Flea110 18:52, 3 January 2006 (UTC)
calving
What time of the year do cows calve?
- I believe this document may answer your question. To quote the document, "There is no single date that is best for the start of calving" Flea110 22:57, 3 January 2006 (UTC)
- In Sweden, cows can calve at any time of the year. The farmer usually tries to put more calvings in the spring though, because in summer you don't have to feed them with hay or silage, but let them eat the grass directly from the field (ie lower costs). Also, in the summer, the milk prices are higher (the milk consumption in Sweden is higher in the summer). TERdON 21:31, 5 January 2006 (UTC)
Photos of the stars from orbit?
Why is it that no photos seem to exist of the stars from orbit or space? I'm not talking about telescopic observations, but rather shots of what the starry plane look like, from the perspective of the human eye, while in space. For example, when the International Space Station or other spacecraft are photographed, space behind them always appears simply black. Is this how it appears to the eye or is this simply a problem of camera lenses? Furthermore, one would assume that, for example, from the dark side of the moon one would have a really spectacular view of the stars, but I have never seen any photographs from this perspective from the Apollo program. What's going on? Does the presence of the sun in space hinder the viewing of stars and turn space into a black blanket? Do the stars only come alive when you are orbiting about the dark side of the earth or the moon? Why are they never photographed?
- I think this was answered before. Light inside the spacecraft reflects off the windows and obsures the relatively dim stars. Low resolution cameras used to take pics of crew aren't well suited to taking pics of star, either. Pics could be taken with a little effort, such as turning off the interior lights and taking along a high res camera, but this seems rather pointless, since we have much better pics from orbital telescopes, like Hubble, and even large ground based telescopes. StuRat 22:23, 3 January 2006 (UTC)
- Well, you don't need a high resolution camera for that, just shading, like the hood of Hubble or the length of a telescope, limiting the view to a small angle. It would, however, help to be on the outside of a spacecraft. The Apollo moonlanders seem an obvious choice for that because the astronauts left the spacecraft, but space walks took place before that. And the USSR could have done this from the very start. Which seems like a logical thing to do because it seems spectacular. But there are two 'buts'. Firstly, what do you photograph when you're out there? The stars? Hell no, the stuff you can't see from Earth, like the Earth from space and the dark side of the Moon. Which brings me to the second 'but'. The USSR photographed the dark side of the Moon (the most interresting part of the 'sky' we can't see from Earth) and the US later sent people around the Moon, who could then see it with their own eyes. Guess which received most attention in the general public. Of course this could be a political thing (maybe the US achievements received equivalently less attention in the USSR). But there's another, much more irritating aspect (going slightly off-topic now). There's a very common flaw in the perception of space exploration; you don't need people for it. In this case just a camera. No manned flight needed. I suppose it's partly the influence of Hollywood that makes people think that for space exploration to be 'real' it needs to be manned. Which is causing a problem for the financing because the people and therefore governments want manned space exploration which is tremendously more expensive than normal space exploration. So loads of allotted money gets wasted on useless stuff, leaving the real research in the cold. DirkvdM 10:12, 4 January 2006 (UTC)
- Go out into the countryside on a clear, moonless, night: your view of the stars is breathtaking. Now take a picture. Do you see stars on it? With an ordinary snapshot, no. You would need to put the camera on a support and take a shot lasting several minutes. If you wanted to photograph something else (e.g. a person) you would use flash; getting the stars in the shot too is possible, but needs some work, and a camera more capable than most. Indeed, if you saw a snapshot taken in space and it had stars along with some other brighter object, I would be very suspicious that it was faked. No stars shows it is real! Notinasnaid 11:44, 5 January 2006 (UTC)
- Banging my head against my keyboard. Astronomy and photography are two of my main interrests and I missed this one! DirkvdM 07:49, 6 January 2006 (UTC)
Protein size - rule of thumb?
I am looking for a rule of thumb on the molecular weight of proteins. Any opinion of the "average" MW of proteins? Or the range of MW that most proteins fall in? I know this is an ill defined question, so don't bother telling me that..... ike9898 22:32, 3 January 2006 (UTC)
- See Protein#Diversity --WS 23:32, 3 January 2006 (UTC)
- A molecular mass of about 40,000 is fairly average. Proteins such as myoglobin and calmodulin are good examples of proteins that do not try to do a whole lot, but they show that a particular biological function can often be accomplished with a 15-20 kDa protein. Many proteins combine several functional domains and can easily be 40, 60, 80, 100 kDa. Many membrane proteins have multiple transmembrane domains plus a cytoplasmic and an extracellular domain. Transmembrane proteins in the range of 80-160 kDa are fairly common. Attempts have been made to identify all the proteins in various organisms. In Mycoplasma hyopneumoniae the average protein size is 388 amino acids (see). The recent article, "Protein length in eukaryotic and prokaryotic proteomes" says that among the Eukaryotic proteins analyzed the average protein was 361 amino acids long while in Bacteria the average was 267 amino acids. --JWSchmidt 00:05, 4 January 2006 (UTC)
- Thank you! Using another rule of thumb (number of amino acids times 110 = estimate of MW):
- 388 amino acids represents a MW of about 42.6 kDa
- 361 """" 39.7 kDa
- 267 """" 29.4 kDa
- ike9898 14:19, 4 January 2006 (UTC)
- Thank you! Using another rule of thumb (number of amino acids times 110 = estimate of MW):
- A molecular mass of about 40,000 is fairly average. Proteins such as myoglobin and calmodulin are good examples of proteins that do not try to do a whole lot, but they show that a particular biological function can often be accomplished with a 15-20 kDa protein. Many proteins combine several functional domains and can easily be 40, 60, 80, 100 kDa. Many membrane proteins have multiple transmembrane domains plus a cytoplasmic and an extracellular domain. Transmembrane proteins in the range of 80-160 kDa are fairly common. Attempts have been made to identify all the proteins in various organisms. In Mycoplasma hyopneumoniae the average protein size is 388 amino acids (see). The recent article, "Protein length in eukaryotic and prokaryotic proteomes" says that among the Eukaryotic proteins analyzed the average protein was 361 amino acids long while in Bacteria the average was 267 amino acids. --JWSchmidt 00:05, 4 January 2006 (UTC)
wind power
what are the advantages and disadvantages of using wind power?
- Have you read the article, wind power? - Fredrik | tc 23:39, 3 January 2006 (UTC)
January 4
Champagne and soft drink bubbles
Where do these bubbles come from it often appears that they start from the middle of the fluid in random positions what governs this? they simply flow constantly from what appears to be a point of no gaseous pressure significance. (7121989 01:55, 4 January 2006 (UTC))
- The carbon dioxide bubbles form from carbonic acid present in both. As for the location of nucleation sites, where the bubbles form, they can be node points on sound waves when you ping the glass, a small particle in the fluid, etc. StuRat 02:19, 4 January 2006 (UTC)
- Very interesting question. As StuRat said, the bubbles form wherever there are particles in the fluid, or on the sides where there are imperfections in the glass. A fun thing to do is put a raisin in the soda. It will become covered with bubbles until it floats up to the top, where the bubbles pop and it falls again. —Keenan Pepper 08:09, 4 January 2006 (UTC)
X-ray machines at airports
1) HOw is it that the X-rays from these machines do not in the sightest damage film from cameras or laptops yet still be strong enough to penetrate luggage?
2) How is a thin strip of plastic able to protect those working around these machines from tissue damage?
3) Finally, When viewing objects being scanned there are usually two screens used by operators, one of a darker image and another in greenish tints what does each one do? (7121989 01:55, 4 January 2006 (UTC))
- The X-ray machines used to scan carry-on luggage are not as strong as the ones to scan checked luggage as the items being scanned are generally smaller and can be more easily checked by hand. This is why the carry-on x-ray does not cause damage to film, electronics or people. The screens are different because one is looking for metal (such as knives or guns) and the other is looking for organic material (explosives, drugs, live animals). See How Stuff Works for a good explanation of airport X-rays. --Canley 02:18, 4 January 2006 (UTC)
- I think also that X-ray machines, both medical and for security, have become much much more sensitive, and so can use much less radiation than they used to. For medical machines, in addition to the obvious radiological benefit, it allows subtler detail to be visible. A few years ago I needed a foot x-ray, and on looking at it you could see the granular structure of my foot bones - it wasn't just white for bone, black for not-bone. That added sensitivity will be vital for looking for those soft things like explosives. -- Finlay McWalter | Talk 02:37, 4 January 2006 (UTC)
- Photographic film isn't really very sensitive to X-rays; it's sensitive to visible light. In the bad old days of very high intensity airport X-rays, people did find fogging on their higher-speed films, but today's moderate or low-intensity scanners don't pose a threat to normal films. (Medical X-ray films aren't directly sensitive to X-rays either; a fluorescent plate is used to convert X-rays to visible light.) But I don't think it's just plastic that's protecting workers and bystanders; I think you'll find it's a plate of lead or other heavy metal. Sharkford 16:59, 4 January 2006 (UTC)
Possibility of X-ray goggles
By X-ray googles i don't mean goggles that emit X-ray's, which i know is odd i simply refer to a (portable) device able to penetrate clothing (and no not just of beautiful yound women, security too!) and reveal an image we can see, is this possible without using harmful high energy waves, if not what alternatives are there which come close? (7121989 01:55, 4 January 2006 (UTC))
- There are many devices that use infrared (heat) cameras. Many clothing items allow heat to pass through. Any item between the body and the clothing will then show up easily. There is a lot of concern about these because show the outline of the body beneath the clothing. Also, pubic hair blocks visible body heat, so it the outline is visible. In the United States, anything related to nudity must be forbidden. So, we won't have publicly advertised infrared security systems anytime soon. As for hidden infrared security systems, they are probably installed and used in many locations already.
- Want to make your own? Get a camera with a cheap pickup. It will have an infrared filter to block the infrared light (that makes the picture fuzzy). Remove the filter that blocks infrared and replace it with one that blocks visible light. Presto - you have an infrared camera. Then, hang out at the pool (where clothing is designed to allow the most heat to pass through), get arrested for taking semi-nude photos, and try to explain that you are just trying to make security better. --Kainaw (talk) 02:29, 4 January 2006 (UTC)
- Cheap CCD cameras are only sensetive to near infrared, and thus are only giving you a view of things that are slightly redder than red (it may see through some fabrics but only ones that pretty transparent to visable light). True heat cameras generally use expensive cooled CCDs. The cutting edge for this security scanning technology is Terahertz radiation. --Martyman-(talk) 07:07, 4 January 2006 (UTC)
This was also asked at the miscellaneous ref desk. Please don't double post. DirkvdM 10:53, 5 January 2006 (UTC)
See Backscatter X-ray - it is effectively a "X-ray goggles". Samw 22:34, 6 January 2006 (UTC)
Help with heat!
Hello, I don't have a calorimetre and I need to find the specific heat of lemon juice and any kind of antacid! I can't calculate specific heat, so If anyone can help me by just telling me that would really help out!!! Thank you all so much! Aberforthbil1657
- Here's a sample equation:
- Calculate the specific heat capacity of copper given that 204.75 J of energy raises the temperature of 15g :of copper from 25˚C to 60˚C.
- q = m x Cg x (Tf - Ti)
- q = 204.75 J
- m = 15g
- Ti = 25˚C
- Tf = 60˚C
- 204.75 = 15 x Cg x (60 - 25)
- 204.75 = 15 x Cg x 35
- 204.75 = 525 x Cg
- Cg = 204.75 ÷ 204.75 = 0.39 J˚C^-1 g^-1
- Sorry, I can't do LaTeX.
- Here's a short guide for the military [16], and Britannica's [17]. Do you know how to do the experiment? --
 Mac Davis ญƛ. 05:50, 4 January 2006 (UTC)
Mac Davis ญƛ. 05:50, 4 January 2006 (UTC)
pain and screaming
What is the reason that people scream when subjected to intense pain? I understand the reasons for pain and its beneficial nature, but the reason as to why people scream when they hit their finger with a hammer, for instance, eludes me. Is there a reason?
--24.29.92.197 02:26, 4 January 2006 (UTC)
- To enlist the assistance of other members of the tribe ("help, I'm being bitten by a lion") or to warn them of danger ("beware: I'm being bitten by a lion"). -- Finlay McWalter | Talk 02:30, 4 January 2006 (UTC)
- Ever wonder if humans scream differently when wanting others to come help or wanting them to run away? --Kainaw (talk) 02:36, 4 January 2006 (UTC)
- Or how about other animals? — Knowledge Seeker দ 06:18, 4 January 2006 (UTC)
- Nah, we animals react in the same way in both cases, we're just programmed to scream, without thinking whether it is meant to attract others for help, or to warn them, or whether it is futile. Pigs/cows, etc. scream out loud in intense pain in slaughterhouses, and I guess humans will do the same, regardless of the fact that it's not going to make any difference. The screaming is not a result of a design, but it just happened that the mutants that screamed had better rate of survival in the natural selection process. And it's a reflex action, it doesn't involve thinking and decision making ("should I request help or should I warn?"). deeptrivia (talk) 06:29, 4 January 2006 (UTC)
earth
i am working on a class project and i want to know how high u can jump on earth--66.38.206.223 02:39, 4 January 2006 (UTC)
- Well, you're on Earth, why don't you try it and measure how high you can jump? You could also look at the records for the sport High jump. See also Jumping and Gravity. --Canley 02:59, 4 January 2006 (UTC)
- How can she know how high you can jump on the Earth unless you tell her? deeptrivia (talk) 15:50, 4 January 2006 (UTC)
- Why do you assume he/she is on Earth? The question only makes sense if he/she has never been to Earth, so doesn't know what 10 m/s2 gravity feels like. =P —Keenan Pepper 08:23, 4 January 2006 (UTC)
- Doesn't the question specifically ask "how high u can jump on earth"? It all depends how you define how high you can jump. Is it the highest from the ground? Then high jump records are what you need to look for, specifically Javier_Sotomayor who has jumped higher than anyone. However, there is also the highest jump above ones head. It currently stands at 59cm. David D. (Talk) 08:30, 4 January 2006 (UTC)
- Why do you assume he/she is on Earth? The question only makes sense if he/she has never been to Earth, so doesn't know what 10 m/s2 gravity feels like. =P —Keenan Pepper 08:23, 4 January 2006 (UTC)
| height above head (cm) |
height of athlete (cm) |
height jumped (cm) | name | nationality | place | date |
|---|---|---|---|---|---|---|
| 59 | 173 | 232 | Franklin Jacobs | USA | New York | 27 jan 78 |
| 59 | 181 | 240 | Stefan Holm | SWE | Madrid | 6 mar 05 |
The high jump as an international athetics event has a rule that the competitor must jump using only one foot. So these records don't tell the whole story: it may be possible to jump higher using both feet. Gdr 12:07, 4 January 2006 (UTC)
- It is not possible to jump higher with two feet than one. In the high jump, the trailing leg is used to provide much of the upwards momentum during 'take off', converting the lateral momentum of the run up into vertical motion. Watch the way the trailing leg kicks during a Fosbury flop. It is nowhere near possible to jump higher using both feet. For one, you wouldn't be able to have a fluent run up, thus there'd be no momentum to convert into upwards motion. Proto t c 12:41, 4 January 2006 (UTC)
- I assume Gdr is refering to the vertical jump test. It is described at the following link as below (note i am not sure how reliable this article is since they cite an incorrect world record for the high jump).
- "The best measure of jumping ability one that does not depend on the jumper's height is the standing vertical jump. The individual stands facing a wall, and with arm extended and feet on the floor, makes a mark on the wall at the top of his or her reach. The person jumps vertically, making a second mark at the peak of the jump. The distance between these two marks measures the vertical leap. This is an accurate measure of leaping ability, as each part of the jumper's body rises the same distance. A typical athlete has a vertical leap of 1 1/2 to 2 feet; the best male jumpers attain heights of 3 1/2 to 4 feet."
- The claimed maximum height for the vertical jump is 4 ft (I could not find a verifiable wordl record) and equates to reaching a maximum height of 122 cm. This is quite a bit lower than Sotomayors 143 cm world record high jump. David D. (Talk) 00:08, 5 January 2006 (UTC)
What is a hepatitis a?
See the search box on the left of the screen? Try searching for "hepatitis" and see what turns up. --Robert Merkel 02:53, 4 January 2006 (UTC)
Solutions
If I have a solution with several components such as milk, water, and ammonia, how can I find the specific heat of the solution? I know the specific heat of the individual reactants but I don't know how to determine the specific heat of the overall solution. If anyone knows please list in J/g degrees celsius, Thanks! johnbog456
- If a chemical reaction is involved, then I guess it's not really possible to get the sp. heat of the products from that of reactants. deeptrivia (talk) 03:24, 4 January 2006 (UTC)
- As long as there is no chemical reaction (ammonia and milk might react; not sure) The specific heat of a mixture is the sum of (the specific heat of each part * the proportion of the mass of each part). Post again if that doesn't make sense to you. (found at http://www.carnicom.com/drought1.htm )
- There seems to have been lots of similar questions lately. --AySz88^-^ 05:44, 4 January 2006 (UTC)
Yeah, that's right. Basically heat capacity (mass*sp. heat)is additive. So, (m1+m2)*C = m1*c1+m2*c2, where C is the sp. heat of the overall soln.deeptrivia (talk) 05:51, 4 January 2006 (UTC)
Cracking a Linux box
I half don't expect and answer to this or I'm afraid I already know the answer. Can I crack the root password on a Linux box without root access? I set one up several months ago and it has been sitting on my network contently doing nothing. Today when I tried to log in as root I found I'd forgotten my password. I tried all the various combinations I might have used to no avail. I still have a regular user but that's about worthless. It's running slakware 9.0 and I've upgraded the kernel to 9.4.22. I figure I might be able to make a boot/root floppy set and run setup. Will that work? Can I do the same from an iso image CD. I'd start searching Google, but I've gotten spoiled by Wikipedia's reference desk. It's not as fast but I don't have to choose from sixteen gizzilion possibilities. Thanks.--◀Pucktalk▶ 04:48, 4 January 2006 (UTC)
- Try booting with
init=/bin/shin the kernel command line. —Keenan Pepper 07:33, 4 January 2006 (UTC)- I'm going to try that with an ISO CD, but I was kind of hoping to do it remotely. I don't have a keyboard or monitor attached and I don't really want to have to lug one into the other room. If I have to go through all that I may just wax the whole thing and put the slakware distro on it, assuming that will even work on a p133. Thanks.--◀Pucktalk▶ 07:54, 4 January 2006 (UTC)
- Hmm, remotely. Let's think about that. If you can get root access without a password remotely, then anyone can, right? =P The reason why the init=/bin/sh thing isn't a security hole is because you need physical access to the box. —Keenan Pepper 08:17, 4 January 2006 (UTC)
- That was kind of what I meant by I'm afraid I already know the answer. Security bites at times.--◀Pucktalk▶ 08:55, 4 January 2006 (UTC)
- Others mentioned local root exploits. If it's been several months and you haven't accessed the machine to apply patches, there is probably a local root exploit you could use to gain access. Basically go to http://www.securityfocus.com/vulnerabilities, pull down linux and kernel or your specific distribution and look for the local root exploits. They're not just going to show you how to do it, so if you didn't already know how to use an exploit you're not going to be able to easily. Better solution is to go get physical access and use a livecd as others mentioned. - Taxman Talk 18:13, 6 January 2006 (UTC)
- That was kind of what I meant by I'm afraid I already know the answer. Security bites at times.--◀Pucktalk▶ 08:55, 4 January 2006 (UTC)
- Hmm, remotely. Let's think about that. If you can get root access without a password remotely, then anyone can, right? =P The reason why the init=/bin/sh thing isn't a security hole is because you need physical access to the box. —Keenan Pepper 08:17, 4 January 2006 (UTC)
- Boot from a rescue CD which gives you write access to the boot partition with root access. If you don't have a suitable CD, then take out the hard disk and mount it in another Linux box. On a Linux box where you know the root password, look at /etc/passwd or /etc/shadow (depending on your configuration) and note the encrypted version of your password there. Then edit the passwd or shadow file on the disk of the system you've forgotten the password for. You now have a known password on that system.-gadfium 08:06, 4 January 2006 (UTC)
- I think the slackware install CD will give write access, if not I know I've got knopix around here somewhere. I do have another box where I know the root password. So what you're saying is if I edit /etc/shadow on the box I'm locked out of so that root has the same hash as the box I can get into that should fix it? That makes sense if I'm understanding you right.--◀Pucktalk▶ 08:55, 4 January 2006 (UTC)
- One other thought: are you trying to login as root at the console? It is likely to be forbidden from remote sessions. It's easy to mistake this symptom as "forgotten password". Notinasnaid 10:25, 4 January 2006 (UTC)
- I vaguely recall that when remote root is disabled you get an message saying so. I rarely disable remote root becuase the boxes are inaccessable to the outside world unless you can get through two NAT boxes. Anyway, I'm logged in as my regular user and trying to su. I've most definitely forgotten the password.--◀Pucktalk▶ 10:37, 4 January 2006 (UTC)
- For completeness, if the box is running old software for which there are local root exploits, you could use that root exploit to get root and reset the password. Note that if you can exploit it, so can anybody else with shell access to the box (or can use a remote exploit to execute that command as an unprivileged user), so fix the hole after you use it. --Robert Merkel 12:54, 5 January 2006 (UTC)
- I vaguely recall that when remote root is disabled you get an message saying so. I rarely disable remote root becuase the boxes are inaccessable to the outside world unless you can get through two NAT boxes. Anyway, I'm logged in as my regular user and trying to su. I've most definitely forgotten the password.--◀Pucktalk▶ 10:37, 4 January 2006 (UTC)
Forgot my user password on Windows XP Professional
On my desktop, I forgot my user password, and the hint word doesn't make any sense to me any more. How can I get into my account again? deeptrivia (talk) 05:22, 4 January 2006 (UTC)
- Take it to a computer repair place, and tell them. That's the easiest way. -- User:Mac Davis
- Yeah, it is, but if you have to do it often, such as when supporting people who can't understand how to use ntpasswd, using it works like a charm every time. It's probably what the guys at the repair place will use. I just wish I had the same thing for my Linux box.--◀Pucktalk▶ 07:47, 4 January 2006 (UTC)
- For Linux, just stick in a Knoppix CD, mount your normal root partition and edit /etc/shadow under it. The install CD for your distro probably also works, you just need to get into a shell somehow (try Ctrl-Alt-F2). —Ilmari Karonen (talk) 12:42, 4 January 2006 (UTC)
- Yeah, it is, but if you have to do it often, such as when supporting people who can't understand how to use ntpasswd, using it works like a charm every time. It's probably what the guys at the repair place will use. I just wish I had the same thing for my Linux box.--◀Pucktalk▶ 07:47, 4 January 2006 (UTC)
- Thanks, that was informative. I'll check ntpasswd out! deeptrivia (talk) 22:31, 4 January 2006 (UTC)
Movement of the Earth
Earth has a bit of information under the subheading Earth in the solar system and there is also some information at Planetary orbit.--Ali K 07:57, 4 January 2006 (UTC)
- And don't forget precession. StuRat 22:46, 4 January 2006 (UTC)
seeking widest possible concensus
I'm seeking a concensus on the subject of mergers at eumetazoa. TheLimbicOne(talk) 14:46, 4 January 2006 (UTC)
shadows caused by albedo
I was wondering if there is a specific term for the shadow created from a reflective body such as the moon. In a sense, we know something is a shadow from a "direct" source of light whether it be natural(the sun) or artificial(a light bulb) but as far as these producing the light to something else and then this specific body reflecting the light: shouldn't there be a different term?
Your input would be much appreciated. I've been pondering this thought for some time now.
Sincerely,
Christopher Cole Chardon, Ohio
- Now you've done it. I'm going to have "Moonshadow" playing in my head all day. Well, at least it's not "My Sharona". --Trovatore 15:47, 4 January 2006 (UTC)
- It took me a long time to understand the question, but I suppose you simply mean "What should one call a shadow that is cast by reflected light?" I don't see why there should be a different term and anyway, most light we see is reflected. Consider what what shadows would be like if the Sun's light weren't reflected all over the place by the atmosphere (or whatever). They would be totally absolutely completely pitchblack. Most of the light around us is reflected light. And I imagine that light that is created in the Sun gets reflected many times there, too, so even our main source of light is mostly reflection (don'\t know this, just seems plausible). DirkvdM 11:03, 5 January 2006 (UTC)
RHEUMATOID ARTHRITIS CURE
(no text in body of question)
- Please read the instructions at the top of the page and try again. - Mgm|(talk) 19:09, 4 January 2006 (UTC)
- I don't believe there is a cure, just ways to treat it. Aspirin, for example, relieves pain and reduces associated swelling. StuRat 22:40, 4 January 2006 (UTC)
element lead
what is the melting point of the element lead?
- I added a few square brackets to your question. Does that help? —Ilmari Karonen (talk) 22:24, 4 January 2006 (UTC)
- (If you're confused, that means "click on the lead link".) --AySz88^-^ 04:34, 5 January 2006 (UTC)
- ...are animals that live in the water and on land. What else would you like to know? -User:Lommer | talk 22:52, 4 January 2006 (UTC)
Energy Conservation 2
If a virtual particle appears in a vacuum, hits another particle, losing energy, and bounces off into it antiparticle, then then wouldn't energy conservation be violated because it has lost some of the borrowed energy? Thanks 216.209.153.49 23:24, 4 January 2006 (UTC) Max
- If you look above, to here, the question has already been answered. GeeJo (t) (c) • 23:43, 4 January 2006 (UTC)
- It's the same guy. -lethe talk 23:47, 4 January 2006 (UTC)
I answered above. -lethe talk 01:45, 5 January 2006 (UTC)
Universal IDs
Greetings:
I'm trying to find material relating to the concept of "Universal ID", which, in my case, is defined using the following scenario:
" A group of government officials and information managers at major corporations who point out that the proliferation of single-use identifying keys for individuals is causing major inefficiency, embarrassing and costly cases of mistaken identity, and considerable waste of time and money. They propose a single lifetime ID for every Canadian resident that would be used for everything from tax returns to grocery check-cashing cards."
And I have to argue along the lines of:
"a universal ID would lead to loss of privacy and essential freedoms, and would be open to considerable abuse."
I would highly appreciate any pointers to wikipedia entries, books, journal articles, web site materials pertaining to "universal ID" in the above sense.
Regards,
129.97.252.63 23:30, 4 January 2006 (UTC)
- I believe Identity card is what youre looking for, but I don't think it examines the Canadian case in great detail. GeeJo (t) (c) • 23:45, 4 January 2006 (UTC)
- I'm not sure how much they've written about this topic in particular, but the folks at the [Cato Institute] would certainly have something opposing it. Similarly, you might want to search [Reason Magazine]. --George 14:27, 5 January 2006 (UTC)
January 5
Lungs
How do the lungs Work?
- Perhaps you didn't realize this is an encyclopedia. Have you tried looking up lung? -- Rick Block (talk) 00:24, 5 January 2006 (UTC)
Oxygen Planet
Ok, in theory if i took a planet the size of earth same atmospherical structure but 100% oxygen consistency, landed on this planet (with a suit) and lighted a match, would the planet exploded? I have been told 'No' by various sources, why not?
And as another interesting point when I light a match on Earth why doesn't 20% or so of the earth atmosphere explode? (7121989 00:50, 5 January 2006 (UTC))
- Oxygen alone isn't an optimal fuel for an explosion. Oxygen atoms will combine with other oxygen atoms to create 02. When you heat up the O2 to break it apart and force it to combine with another oxygen atom, you don't get much heat surplus. Now, if you use a molecule that is a little unstable, provide it with something it wants (usually oxygen), and then add heat to give it a nudge, the molecule will break, release a lot of heat, and the halves will combine with the oxygen. So, all in all, you really need fuel, heat, and oxygen to get something good going. Consider going to a planet that has an atmosphere of well mixed 50% oxygen and 50% methane - then light a match. Of course, I doubt you'd ever get the chance since some flaming meteorite will beat you to it. --Kainaw (talk) 00:59, 5 January 2006 (UTC)
- Of course the planet's surface might also be flammable, but then the same would apply - a meteor would probably have beaten you to it. Other than that there are two (potential) fuel sources. First there is the match, which will burn up incredibly fast - you'll see small 'poof' and then nothing. The second source is you. Better make that a very non-combustible suit or the little 'poof' will be followed by a somewhat bigger one... DirkvdM 11:11, 5 January 2006 (UTC)
- Of course, the heat of atmospheric entry would have blown the entire place up even if a meteor hadn't wandered along yet. 198.62.217.2 13:11, 6 January 2006 (UTC)
A new form of fire?
What other examples are there of fire, in the sense of rather than rapid oxidisation, an exothermic reaction with another element or compound?
With these examples could one make a new type of more efficient combustion which can remain more prolonged?
Or, just came up with this the other extreme, cold fire, an rapid endothermic reaction which emits cold as it sucks in lights and sinks to the ground like a really cold dark heavy smoke, is it possible to be created? (7121989 00:50, 5 January 2006 (UTC))
- Similar to the question above, your question is limited to oxygen combustion. There is nuclear fusion and fission also - neither of which requires oxygen and both are capable of being prolonged for very long times. However, I cannot think of any chain reaction that absorbs energy. You might try to call a black hole a chain reaction that absorbs energy, but I'd say you are being very liberal with the idea of a chain reaction. --Kainaw (talk) 01:01, 5 January 2006 (UTC)
- There really can't be any other form, since that is the definition of fire. An endothermic reaction like your "cold fire" is not self sustaining and won't have the power to suck in light, something that only black holes can do. Matter can absorb light or heat that hits it directly, but won't "suck it in." There's no such thing as emitting cold, since cold is a lack of heat. An endothermic reaction like the one inside a chemical cold pack will absorb heat from the environment, but it won't necessarily be dark, nor will it exist in the form of smoke or have a particular density. Night Gyr 01:10, 5 January 2006 (UTC)
Rust is oxidation of iron. Is it exothermic? User:Zoe|(talk) 16:41, 5 January 2006 (UTC)
- I believe it is, but it happens at such a slow rate that the temperature difference generated is negligable. -User:Lommer | talk 17:58, 5 January 2006 (UTC)
Most expensive chemical element and compund
What is the most expensive chemical element and compound by market price currently? and i have also heard ridiculous prices on antimatter, but has it ever been made, surely none exists now? (7121989 01:34, 5 January 2006 (UTC))
- Bear in mind, the most expensive elements are all only available in very, very small quantities, usually as the result of high-energy collisions in particle accelerators, and aren't feasibly producable in reasonable quantities. As such, any cost-figure attached to elements are simply scaled up from the cost per atom. Because of this, any element of about 110+ is going to be hideously, mind-bogglingly expensive. As for antimatter, yep, it's been produced. It's used in PET scans among other things, though again, only on the order of a relative handful of particles. GeeJo (t) (c) • 04:11, 5 January 2006 (UTC)
- What's the most expensive stable element? Common Man 03:04, 6 January 2006 (UTC)
lizard regeneration
Is it possible for a new lizard to grow from a piece of tail broken off an original? I know that it can grow a new tail if it loses one, but can one grow from the piece that has fallen off?
Salamanders are better than lizards at leg regeneration. The "fallen off piece" does not grow a new salamander. For an up-to-the-minute review of limb regeneration and how some of our new understanding may be applied to human organ regeneration, see this week's issue of Science: [18] alteripse 02:19, 5 January 2006 (UTC)
- While lizards can't do that, note that planaria, a type of flatworm, can do so. StuRat 10:26, 5 January 2006 (UTC)
- Also, the Crown-of-Thorns starfish, a major blight on Australian reefs, can do this. The method of population control used was to hack them to bits and throw the bits into the ocean, an unfortunately each of the little bits grew into a new starfish, increasing the numbers dramatically. smurrayinchester(User), (Talk) 15:43, 5 January 2006 (UTC)
Earths location
Lets say the Earth was 1 foot closer to the sun as it is now...how much would that affect the climate here in Earth?— Preceding unsigned comment added by 68.117.16.64 (talk • contribs) 2006-01-05 05:32:13 (UTC)
- Go outside and stand in the sun. Then get a stool and stand on that a foot higher. Any difference? The Earth's orbit wobbles a bit and has been speculated to cause changes in climate, e.g. the ice ages, but that's likely to be more than a foot. enochlau (talk) 05:51, 5 January 2006 (UTC)
- Interesting question. The Earths orbit is elliptical so as it goes round the sun at certain times of the year it is closer than at other times. The 1 foot you mention is a trivial difference. At its closest distance to the sun, perihelion, the Earth is 147 million km from the Sun. At its greatest distance, aphelion, it is 152 million km from the Sun. So there is a difference during the year of approximately 5 million km. The Earth is closest to the Sun in
JulyJanuary and furthest away inJanuaryJuly. David D. (Talk) 05:52, 5 January 2006 (UTC)
- Actually this year Earth was at closest, perihelion, on Jan 4 (yesterday), it will be farthermost away, aphelion, July 3.USNO Data.--◀Pucktalk▶ 06:07, 5 January 2006 (UTC)
- To demonstrate how insignificant this difference would be, let's do some calculations. The amount of light which hits the Earth from the Sun is inversely proportional to the square of the distance. Since the average distance is around 93 million miles, this is about 491 billion feet. The difference in the squares of 491 billion feet and 490,999,999,999 feet is about 0.0000000004%, so it would change the Earth's temp by about that percentage. This would work out to around 0.000000002 degrees F. These calcs are very approximate, but give some idea of how insignificant the change would be. StuRat 10:06, 5 January 2006 (UTC)
- Of course the Earth's orbital period would have to decrease too, but even if there is an exponential relation between the two (how do they relate again?) then that would also be too minute a change.
- But how much would the distance have to change for it to have any effect? And suppose global warming would really get out of control, could we use nuclear explosions (like in the tv series Space: 1999) to achieve this? And while we're at it, alter the rotational period too, so we get 100 or 1000 days per year, so timekeeping can be made nice and decimal too. :) DirkvdM 11:33, 5 January 2006 (UTC)
- Use nukes to move the earth? No, no ... no. All of about 0% of an explosions energy (no matter how big) affects the momentum of the planet. The most effective way to move the planet would be to sling HUGE amounts of matter in the opposite direction, at high speed. A Moon-sized chunk should get you a few percent further out from the Sun, if you could get it faster than ~11km/s (escape velocity). And then you'd have to make sure its orbit won't ever intersect the Earth's new orbit, or KaPOW! Tzarius 23:09, 5 January 2006 (UTC)
- In case one is wondering how the Earth can be closer to the Sun in winter, the answer is that the effect of Earth's axial tilt completely overwhelms any difference made by the varying distance. Plus, it's summer south of the equator. -- Cyrius|✎ 07:51, 6 January 2006 (UTC)
How to copy/paste wikipedia articles including images
To make a long story short, I would like to be able to copy and paste entire wikipedia articles (including text and images) onto my usb drive so that I can take those articles and view them on a different (offline) computer. When I select everything on a wikipedia article and copy and paste it into a microsoft word document, all I get is text, no images. If I individually select the pictures and copy/paste them it works fine, but that would take an extremely long time. How can I do this efficiently? (Please note that I have no intention whatsoever of using this for any illegal or immoral reasons) Flea110 06:53, 5 January 2006 (UTC)
- If you're using IE, have you tried File --> Save As... ? The resulting files are somewhat messy and inefficient, but it might work, and it's fast and easy. --AySz88^-^ 06:59, 5 January 2006 (UTC)
- I'm actually using firefox, and I just tried using the 'save page as' function. I saved the page to the desktop, and when I opened that file (still on the first computer), the page was a little garbled (but certainly readable) and the pictures showed up fine. When I transferred the file to the offline computer, the text showed up fine, but the where the images should have been there were little red "x"s in the corners, and it displayed the text name of each image. Can anyone think of anything else? (thanks for trying, aysz88) Flea110 07:13, 5 January 2006 (UTC)
- That's because IE bundles the html file, the css file (if present), the image files, and possibly other used files into one file on your harddrive (a .mht file, multipart html file). So when you copy it to the other computer, everything works fine. Firefox, unfortunately, DOESN'T do it. It puts all files except the HTML file in a separate folder next to the HTML file. If you want to transfer it to another computer after that you'll have to copy both the HTML file and the folder. It's a pity really - the MHT feature is one of the few REALLY GOOD features of IE, I think. There seems to be a Firefox extension to fix the problem and add the feature to FF though, but I haven't tested it. TERdON 21:52, 5 January 2006 (UTC)
I actually just received the answer to this question from a friend via msn messenger. In case anybody else is wondering, here's how it's done. File--> Save As, (be sure 'web page, complete' is selected) and save it DIRECTLY to the usb drive. It works fine then. aysz88, you were right after all, but my mistake at the time was not saving it DIRECTLY to the usb drive. Ahh.. Feels so good to solve a problem. Flea110 07:46, 5 January 2006 (UTC)
- Glad you got the answer to your problem. :) --AySz88^-^ 07:56, 5 January 2006 (UTC)
- An alternative would be to use HTTrack, a very powerful tool (so be careful what you do). It will not only download the page but also all the pages it links to. In case of Wikipedia this would download the whole site because everything is ultimately interlinked. Actually, without restraints, it would download the entire internet. To only get the pics you can limit the linkdepth to one and specify only image links (or something similar - I haven't used it for a long time). DirkvdM 11:45, 5 January 2006 (UTC)
astrology
Is the prediction by astrology correct? Is the basis of astrology is firm?
- Answer to first question is sometimes. Answer to second is no. David D. (Talk) 07:09, 5 January 2006 (UTC)
- The predictions seem correct because typically a horoscope contains four or five vague "predictions" (You will meet a stranger, you will face challenges today). According to probability, at least one of these should come true in some way, and since people want to believe the horoscope, they will see minor coincidences as fulfillment of the prophecy. smurrayinchester(User), (Talk) 08:16, 5 January 2006 (UTC)
- Let's define our terms here. Without necessarily wanting to add credence to astrology, there is a huge difference between the "astrology" advice columns in the daily newspapers which use Sun signs only, that apply to 1/12 of the entire population; and a serious natal chart drawn and interpreted by a professional astrologer that uses an individual's precise birth data and which applies to that person alone. The latter is astrology (whether you think it has any validity or not), the former is not. JackofOz 08:33, 5 January 2006 (UTC)
- And I stand by my answer above. Smurrayinchester is correct when he says that people want to see it work and will shoe horn the predictions into known events or cherry pick their character traits to fit. For those with a less cynical view I suspect it is often self fulfilling (obviously this is a POV comment). In this sense it may be comforting and even positive with regard to ones life. David D. (Talk) 08:41, 5 January 2006 (UTC)
- I wouldn't dispute any of that. I'm just saying that, mostly, what people think of as "astrology" is a misnomer, it's something that has virtually no relevance to true astrology at all. JackofOz 08:51, 5 January 2006 (UTC)
- Oh, maybe it was not clear, i agree with your comments. May be I am missing this too. I thought the astrologers were all about prediciting character and to a certain extent fate. Certainly I would not call the daily horoscopes astrology, although i thought they did 'pretend' to make some similar statements but there is just no method i.e. fiction David D. (Talk) 09:00, 5 January 2006 (UTC)
Also note that the "theory" behind astrology, if it can be called that, is based on the actions of Greek and Roman gods. If you don't believe in Roman gods, like Mars/Ares, you shouldn't believe that people born when Mars is visible will have the traits of that god (combative, for example). There is a basic incompatibility in believing in Christianity or any other modern religion and also believing in astrology. There is also a basic incompatibility in believing in science and astrology. StuRat 10:22, 5 January 2006 (UTC)
- This is not true at all. Astrology was never so limited and many Christians (and scientists) were astrologers throughout the early modern period. Our history of astrology does a fairly good job of outlining that. A belief in astrology does not necessitate a belief in Roman or Greek mythology, it simply requires the believe that "heavenly bodies" could have influences on individual people. The place where astrology and Christianity usually butted heads was the issue of free will, not in gods. --Fastfission 03:12, 6 January 2006 (UTC)
See Forer effect. --Robert Merkel 12:47, 5 January 2006 (UTC)
dandruff
how to cure dandruff
- Read the article Dandruff. There's a section on Treatment. --Canley 09:10, 5 January 2006 (UTC)
agriculture
How can science help agricultutre?--~~how science can help agriculture?--219.94.50.118 10:37, 5 January 2006 (UTC)
- Is this, by any chance, homework? Notinasnaid 11:37, 5 January 2006 (UTC)
- Must be a very important question, because (s)he asks it twice. DirkvdM 11:47, 5 January 2006 (UTC)
- Here's a hint to start you off when you do your own homework: agricultural science. --Robert Merkel 12:48, 5 January 2006 (UTC)
computers
what is an operating system? who is considered the father of modern computers?
- See Operating system. As for the father of modern computers, depends on what you call a modern computer and whether you're thinking about hardware or software. Blaise Pascal and Charles Babbage constructed mechanical computers. Ada Lovelace is said to be the first programmer (in which it would be a 'mother' - you male chauvinist pig :) ). If by 'modern computer' you mean the use of transistors then it could be Julius Edgar Lilienfeld (funny, I thought the transistor was a British invention 'stolen' by the Japanese). If you mean the third generation computers, with integrated circuits, then it would be Jack Kilby. See also History of computing hardware. DirkvdM 12:06, 5 January 2006 (UTC)
- Some other luminaries include Alan Turing, who in large part founded computer science, Claude Shannon, who developed digital circuits, J. Presper Eckhart and John William Mauchly for the ENIAC and the design of the stored program computer, John von Neumann for writing up Eckhart and Mauchly's work and circulating it, and Konrad Zuse, for apparently doing most of this independently in Germany before the collapse of Nazi Germany interrupted his work. But the modern computer was the product of many people developing a lot of technologies and figuring out how to combine them, working in collaboration and sometimes in competition, and it's simply impossible to assign one person credit for it all. --Robert Merkel 12:43, 5 January 2006 (UTC)
- Thus I have read: "The Atanasoff-Berry Computer was the world's first electronic digital computer. It was built by John Vincent Atanasoff and Clifford Berry at Iowa State University during 1937-42." This would be without transitors. Tubes and/or relays , I guess. see http://www.cs.iastate.edu/jva/jva-archive.shtml John Atanasoff --GangofOne 00:55, 6 January 2006 (UTC)
- Yes, you can Atanasoff to the list, but the ABC wasn't a modern "computer" as we would understand it. It was a major development that pioneered a number of key features of modern computers, but it was a special-purpose machine as distinct from the later ENIAC and the von Neumann machines that followed it. --Robert Merkel 03:46, 6 January 2006 (UTC)
- Thus I have read: "The Atanasoff-Berry Computer was the world's first electronic digital computer. It was built by John Vincent Atanasoff and Clifford Berry at Iowa State University during 1937-42." This would be without transitors. Tubes and/or relays , I guess. see http://www.cs.iastate.edu/jva/jva-archive.shtml John Atanasoff --GangofOne 00:55, 6 January 2006 (UTC)
- Some other luminaries include Alan Turing, who in large part founded computer science, Claude Shannon, who developed digital circuits, J. Presper Eckhart and John William Mauchly for the ENIAC and the design of the stored program computer, John von Neumann for writing up Eckhart and Mauchly's work and circulating it, and Konrad Zuse, for apparently doing most of this independently in Germany before the collapse of Nazi Germany interrupted his work. But the modern computer was the product of many people developing a lot of technologies and figuring out how to combine them, working in collaboration and sometimes in competition, and it's simply impossible to assign one person credit for it all. --Robert Merkel 12:43, 5 January 2006 (UTC)
Pure copper 3,000 yrs ago
What procedure would people in the bronze age have used to produce pure copper from copper oxide?
- From about 5000BC, smelting, usually charcoal-fuelled, was used to process the ores into pure copper. GeeJo (t) (c) • 13:52, 5 January 2006 (UTC)
Particle accelerators
Hi just wondring if anyone could help me on this. How has the UNILAC accelerator been used to increase our knowledge about chemical elements? How does the technique work and how does it rely on an understanding of the structure of atoms? Thanks
Why is science such important in our daily life?
- See the articles UNILAC, Linear particle accelerator and Science. --Canley 22:12, 5 January 2006 (UTC)
Eye Strain
Is it possible to strain your eyes by reading or watching tv with little or almost no light? It is an old wive's tale that I would like to know the rationality and/or proof behind. Any help will be greatly appreciated! -- Chloe
- How Stuff Works has a pretty good explanation on the subject. GeeJo (t) (c) • 14:06, 5 January 2006 (UTC)
Thanks! That was very helpful! any other sources are still greatly appreciated. -- Chloe
- That article was about reading in low light. Reading is done at close range, which means it requires your eyes' focusing muscles to work harder. (Unfortunately, the article "Accommodation (eye)" is a stub.) Watching TV, you're not sitting so close to the screen, so this is less of an issue. (And at a movie theater you're even farther from the screen and your eyes are practically focused at infinity, so being in the dark matters even less.) -- Anonymous, 21:20 UTC, January 5.
- Also, the brightness on the TV should be adjusted to match the brightness of the room. A dim TV in a bright room causes as much eye strain as a bright TV in a dim room. StuRat 22:13, 5 January 2006 (UTC)
Speed of a horse
How fast can the average horse run? --163.153.132.5 14:42, 5 January 2006 (UTC)
- For a sprint (100 yards)? Or for miles? Notinasnaid 16:15, 5 January 2006 (UTC)
- I don't know much about horses, but the fastest horse in the Melbourne Cup last year ran 3200 m (10 500 ft) in 3m 19 sec. That's an average speed of 58 km/h (36 mi/h). Average horses must be a bit slower than that.--Commander Keane 23:15, 5 January 2006 (UTC)
- According to a quick google search(animals speed):
- A normal 'quarter horse' can run 47.50mph (top speed) for a quarter mile
- A ridden horse can go 40mph
- The distance record for a horse may be 100 miles in 9 hours
- Also, it turns out lots of people use the exact same chart of animal speeds. Black Carrot 02:56, 7 January 2006 (UTC)
- According to a quick google search(animals speed):
Teaching myself Geology
I am teaching myself geology for a course and i am looking through some old exams, some help please i am lost on this q. What name is given to the process which causes surface layer rock to break off? --15:33, 5 January 2006 (UTC)
- I don't believe you; you're eroding people's patience. Proto t c 15:48, 5 January 2006 (UTC)
- I believe we can weather it. Weathering = breaking up of rock into smaller particles. Erosion = movement of those particles from one area to another. This should be in even the most basic geology/Earth science texts. TheSPY 15:59, 5 January 2006 (UTC)TheSPY
erasables( computer storage devices)
- RAM / ROM ? Tzarius 22:49, 5 January 2006 (UTC)
- Or WOM of course :) GeeJo (t) (c) • 02:28, 6 January 2006 (UTC)
astatine
How many protrons does the element Astatine have? How many nuetrons does the element Astatine have? Thank you Amy T207.118.208.184 23:37, 5 January 2006 (UTC)
- From Astatine: The atomic number gives you the number of protons in an atom; in this case it's 85. The atomic mass gives you the total number of protons and neutrons; in this case it's 210. To find the number of neutrons, subtract 85 from 210. Sometimes there are many common isotopes of the element, in which case the number of neutrons would vary; in this case, it looks like there's only one common isotope. enochlau (talk) 23:41, 5 January 2006 (UTC)
How many electrons does the element astatine have? Thank you Amy T207.118.208.184 23:50, 5 January 2006 (UTC)
- As many as it has protons. (Unless it's ionized, in which case it may have more or less.) —Ilmari Karonen (talk) 01:37, 6 January 2006 (UTC)
January 6
Size of internet, size of wikipedia
If you were able to download the entire internet, how much space would it take up on a (rediculously massive) hard drive? How about if you were able to download all of wikipedia? Flea110 01:13, 6 January 2006 (UTC)
- Don't know about the entire Internet, but for Wikipedia, see Wikipedia:Database download. —Ilmari Karonen (talk) 01:34, 6 January 2006 (UTC)
- I thought wikipedia was the whole thing. -lethe talk 02:28, 6 January 2006 (UTC)
- Wow, it's mindboggling! deeptrivia (talk) 02:34, 6 January 2006 (UTC)
- I thought wikipedia was the whole thing. -lethe talk 02:28, 6 January 2006 (UTC)
- The Internet Archive Wayback Machine is about 1 petabyte in size and is growing at a rate or 20 terabytes a month according to its FAQ [19] (compaired to a mere 40 gigs for a wikipedia database dump). The Wayback is presumably larger than the internet because it includes multiple versions of each site. Then again, because its all accessable online its really part of the internet itself, making the actual size of the internet somewhat larger. If you were to include the size of all such caches, including search engine indexes, not to mention material available through filesharing networks and Bittorent, and twenty-some years of newsgroups, the number quickly becomes astronomically large.
- Or for a more succinct response, a quick googling yields this...[20]. Jasongetsdown 03:22, 6 January 2006 (UTC)
- Since 1 GB of hard disk space costs about half a € storing Wikipedia would cost about 8 €. Storing the Internet would however cost half a million €. DirkvdM 08:13, 6 January 2006 (UTC)
- New Scientist found a website which offered the entire web for download at 22.2877482 petabytes, or 23 931 287 382 megabytes [21]. Trying to download it however resulted in "Insufficient memory on drive C: for the internet. Insert disc in drive A". smurrayinchester(User), (Talk) 17:02, 6 January 2006 (UTC)
- Don't forget that the internet is much bigger than just the web. While the web may have lots of web sites and images on it, the internet at any moment has a whole lot of other bytes flying around, including emails, IMs, P2P traffic, etc. That is to say that the more-static web is a tiny fraction of the more-dynamic internet. I recall hearing an ISP study that showed that WWW (web) traffic was actually less than 20% of their traffic. --Quasipalm 19:41, 6 January 2006 (UTC)
Digital cable to DVD Recorder
Can i hook a Wireless Transmitter to my Digital Cable and then hook my wireless receiver to my DVD recorder to record things from Digital Cable?
- Generally, no, because digital TV is always encrypted to stop people accessing channels they do not pay for. This has the side effect that you can't get the raw digital video feed out of the set top box. See Television encryption for more details on this. You can attach a TiVo type device to a digital TV box because it uses an analog interlink. So, you could build a device that takes an analog signal from your digital cable box, re-encodes it as a compressed digital video stream, sends it to a computer which has a DVD burner in it, and then burn it to that. But this would be quite a significant feat of DIY home engineering ;) If you want to record programs from your digital cable box, a PVR like TiVo is still the best way to do it, alternatively I think some home DVD recorder devices can be connected directly to your box like VCR tape recorders could be. Hope that helps -- Anonymous Guy
NSAIDs for dogs
My dog is taking an NSAID called carprofen (no article yet). Why shouldn't dogs take human NSAIDs like aspirin or ibuprofen? Why shouldn't people take carprofen? —Keenan Pepper 02:57, 6 January 2006 (UTC)
- All I can tell you is what a Google search turns up, so take it with a grain of salt. Carprofen has apparently gone through some human trials in Europe, but was never put on the market for economic reasons. Dogs can be given aspirin, but the toxic doses are relatively low and ulcers are likely. Veterinary aspirin is available. Ibuprofen causes stomach ulcers far more readily in dogs than in humans. -- Cyrius|✎ 07:46, 6 January 2006 (UTC)
Free circuit simulator software
I'm looking for a compreensive circuit simulator that can manage things like spark gaps, flyback transformers, an arbitrary number of inductive couplings, etc. I found a nifty one in Java but it doesn't support flyback circuits, and the controls are rather annoying to deal with. The program I'm looking should run on Windows XP.
Anyone knows of such a thing? ☢ Ҡieff⌇↯ 03:49, 6 January 2006 (UTC)
blogs
Blogs? Wouldn't that be better on the humanities reference desk? ☢ Ҡieff⌇↯
Would my ISP be able to know what websites I visit
Would my ISP be able to know what websites I visit? I am in a very small town and there are only two companies offering services here. One ia a big telecom company which I at present use. But, the other company is a new, small and a local company which has given some 50 connections in my town. I plan to move to the small ISP because it is cheap. But, I am afraid whether they would be able to see what websites I visit. Can anyone please tell me? Do you have any other advice or tips?
- Yes, they could, but they probably won't unless you get involved in something which would result in the police requesting the information from them. If they got a website, try reading their Privacy Policy. If I remember correctly, employees are not allowed to look up such info unless there's good reason to. - 131.211.210.11 08:24, 6 January 2006 (UTC)
- Your ISP can potentially see everything you do (except when visiting secure web sites, where they can see where you go, but not what you type). Your e-mails too. In some places they may be allowed to record this information. In some places they may be required to do this, in case the police later want to investigate something. The solution is to not visit illegal sites, I guess. Notinasnaid 10:14, 6 January 2006 (UTC)
- on a side note visiting certian websites will probably get you put on an FBI watchlist, doesn't take much these days, certianly wikipedia users are all on such a list, very subversive website, grounds for concern--172.174.71.47 14:25, 6 January 2006 (UTC)
- Heck using the phrase 'FBI watchlist' on the internet is probably enough to get you on such a list--172.174.71.47 14:26, 6 January 2006 (UTC)
- We've got our Eye on you. And you. - Taxman Talk 16:36, 6 January 2006 (UTC)
You could also get an encrypted connection to a proxy service and use some sort of onion routing or freenet type thing from there. Not perfect, but does make it hard to tell where you are visiting. - Taxman Talk 16:38, 6 January 2006 (UTC)
- I would suggest Tor, being widely supported free / open software. No proprietary protocols or single business in control. Tzarius 06:07, 7 January 2006 (UTC)
Electrophysiology
I am an electronics engg student. My interest is to study about a subject that links electronics with biology or rather human physiology. Is it apt for me to do my higher studies in "ELECTROPHYSIOLOGY"? If yes plz let me know about the books i've to refer to and the universities in the U.S.A and the U.K. which offer this course. --210.214.157.86 09:01, 6 January 2006 (UTC)
- UCAS, who have a list of all the courses available in the UK, don't recognise "Electrophysiology". However, they do have several courses for cybernetics: [22]. Doubtless the US has similar. smurrayinchester(User), (Talk) 17:27, 6 January 2006 (UTC)
- A quick read of our article on electrophysiology might also help. At UCLA we had an undergraduate program in biomedical engineering which sounds like it may be of interest to you. I'm sure there are other schools with similar degree programs as well [23]. --David Iberri (talk) 22:12, 6 January 2006 (UTC)
aromol?
Can someone Please tell me what Aromol is? It is in Smith's Rosebud Salve and I want to know what it is? I have looked everwhere and can't find anything on it? So please someone help me, what is Aromol? Thanks
- You could always try contacting the manufacturer and asking them. --Anonymous, 10:00 UTC, January 6, 2006
optics - experiment
how to make an achromatic doublet ? mail answer to : [email removed]
- Flint glass and crown glass can be replaced by any two materials with different dispersion. —Keenan Pepper 13:35, 6 January 2006 (UTC)
- Also, if you look at our chromatic aberration article, you will see how to calculate the focal lengths of each part of the lens you need, if you know some data about the materials. --Bob Mellish 16:11, 6 January 2006 (UTC)
viral/ infectious diseases
Are there any viruses, bacteria, etc., that live in the cold weather? I know that the cold weather just weakens the immune system and makes the body more succeptible to infection, but i was just wondering whether or not there are any viruses that actually just live in the cold environments and are strong enough to infect people. Thanks! ----- Eryn
Short answer: yes. Long answer is more complicated. There are bacteria and viruses that thrive in extreme environments: freezing and boiling hot environments, but these rarely affect (or infect) humans. Shall we assume that you are only interested in bacteria and viruses that can cause human disease and the degree of cold is the winter temperature range away from the poles where most of us live (like down to 10 degrees below water freezing)? Moderate freezing cold will kill many bacteria and some viruses, but the main effect of cold on bacteria is just to slow down reproduction and activity (which is why refrigeration retards bacterial growth). Bacteria and viruses vary greatly in their abilities to survive outside a host but the temperature is less of a factor in this than availability of water and food and absence of harmful substances like soap or high osmolality or intense sunlight. Dehydration will kill most bacteria faster than cold will, but some viruses can survive dehydration and some can survive indefinitely being frozen. Some pathogenic bacteria require direct person-to-person contact (e.g., bacteria of gonorrhea or the AIDS virus), but others (e.g., the spores of tetanus) can survive in the environment for long periods of time in various forms. For example, there has been concern about whether smallpox or influenza viruses can remain infectious in graves. The most recent example was the investigation a few years ago of 1919 flu victims buried in the permafrost of extreme northern arctic islands for 80 years. Precautions were taken to avoid releasing potentially infectious material. That said, I don't know of any cases of smallpox or plague or influenza known to have been contracted from graves or crypts. Cold weather does have an effect on transmissibility of respiratory viruses by affecting human behavior and perhaps altering mucous membrane defenses. Complex topic. alteripse 13:45, 6 January 2006 (UTC)
Thanks... I know this is a complicated question. You were very helpful. However, are there any specific instances in which entering a cold environment would promote the spread and possible contraction of a virus or bacteria into a human body? And if so, what are they???? ----- Eryn
Ah, this sounds simple, so I'll wade in. This site [24] explains the #1 myth, ie. if you go outside 'You'll catch your death of cold!', which is usually uttered by an old lady in a Jane Austen novel. --Zeizmic 18:05, 6 January 2006 (UTC)
Thanks for your input. I think i worded my second question poorly though. Are there any bacteria, viruses, etc., that live only in the cold weather and are then contrsacted by humans or animals? For example, a virus taht lives and thrives outdoors in cool temperatures and then infects the first host that it encounters. possibly this bacteria/virus stayes dormant until contact with a host is made.... (Maybe this has been answered already in a previous reply and i just dont see it.) But if there is such a virus/bacteria, a name or description would be most helpful. Thanks! ----- Eryn
- I asked our disease control doctor here (MUSC hospital). She said that there are many bacteria and viral-like lifeforms documented in the South Pole. In her opinion, it is heat that is harmful to them, not cold. They usually freeze and thaw well. They overheat and die easily - which is why running a fever is a good thing. --Kainaw (talk) 20:39, 6 January 2006 (UTC)
Physicists
Hi, I read in several places that the fundamental building block of the universe is information or events...I know how it sounds, but I haven't read it in new agey looney pages, I read it in like, news articles, and in some physics pages which I cannot remember,but I can't distinguish real science from far fetched claims...so, do you think this is true or somewhat true? because I am aware that the building blocks of matter are quarks and subatomic particles like gluons and stuff.
also, quite apart from that, here's this quote :We are now synergetically forced to conclude that all phenomena are metaphysical; wherefore, as many have long suspected — like it or not — 'life is but a dream.' - Buckminster Fuller. see? I mean, stuff like this... what do u think of what he says?, or am I taking it too litarally and he meant something else.--Cosmic girl 14:35, 6 January 2006 (UTC)
- Comment: I think, apart from subtle philosophical difference, information in this context can mean energy of which matter is another manifestation, and events can mean time. Space is left out, but again, some people think space it no existence independent of matter. I find this statement a bit vague though. deeptrivia (talk) 16:02, 6 January 2006 (UTC)
- Comment2: Well, I don't know the context, but he's probably referring to something related to the wave-particle duality and uncertainty principle. For more about the "life is a dream" proposition, see Advaita_Vedanta#Advaita_and_Science, Advaita_Vedanta#Three_levels_of_Truth, Advaita_Vedanta#Are_the_world_and_God_wholly_false.3F. deeptrivia (talk) 16:12, 6 January 2006 (UTC)
Thank u :), but what I actually meant to ask was if this notions are somewhat supported by current respectable science? or just by speculation, like eastern philosophies.--Cosmic girl 16:21, 6 January 2006 (UTC)
- Yeah, this wasn't intended as a reply, just some comments. Advaita_Vedanta#Advaita_and_Science should give an idea though of how far physicists entertain these views. deeptrivia (talk)
- I've seen similar scientific speculations, in particular in the book Information - The New Language of Science by Hans Christian von Bayer. As I understand it, the basic idea is to think about a hypothetical perfect theory of everything -- what if you knew all the equations governing reality at the lowest level, and had all the data about everything (knew the full quantum wavefunctions etc), then you could imagine the whole universe evolving as a computational process -- in other words, we don't need to assume any reality beyond the information process. What I got from Bayer's book is that physicists are finding applications for information theory in physics, so that the physical information encoded in a system of particles may in fact have real physical meaning. The traditional basic connection is between entropy in statistical physics and information theory. But I'm no expert on any of this - I can't tell if these physicists have simply confused their models with reality. 84.239.128.9 19:09, 6 January 2006 (UTC)
cool, so you mean that, information theory implies that nothing outside or besides the universe is required for the universe to exist? I mean, nothing besides the information and computations of our universe?... if so, how can it know that? I mean, can't the universe be like a big videogame? it can seem the only thing for us, but we can never know that which lies outside the computer that contains it ... maybe we can only know the software... it sounds really crazy and hard to understand, but i think that the information theory has space for a videogame conception of reality, or a simulation for that matter, but I know nothing about physicists, so I need the expert's opinion. --Cosmic girl 19:30, 6 January 2006 (UTC)
- The expert is just another character in the video game. But anyway, take a look at Edward Fredkin, Steven Wolfram; they attempt to show reality is a large computation in some machine. GangofOne 03:04, 7 January 2006 (UTC)
who are the five google billionaires?
who are the five google billionaires?
- Omid Kordestani, David Drummond, Shriram Kavitark Ram, Sergey Brin and Jonathan Rosenberg --Quasipalm 19:30, 6 January 2006 (UTC)
A query regarding Pepper Pad
Have anyone of you used a Pepper pad? I am just interested in buying a pepper pad, but want to know this- I heard that its resolution is 800x600.
I just want to know how would a 8.4" screen placed horizontally compare with a screen placed vertically with respect to size. Would the 8.4" Pepper pad be equialant to a 15" CRT monitor in 800x600 resolution or would it be equivalent like viewing a 14" CRT screen? Or would it be equivalent to viewing some other screen with someother resolution? Can you please tell me the equivalents?
Can we view full page in a Pepper pad without sideways scrolling?
- Thanks for making me look that up [25] That was really interesting! This seems to be the legendary Linux PDA that everybody has said will come one day. The resolution is good enough to get in most web pages without scrolling (just set your computer to this and see!). The specs look good, but you really need to get some independent reviews. Never be the first on the block! I find the info a bit misleading, since they seem to be marketing to the 'ipod' generation, with all sorts of promises, like browsing in your car. This thing only has Wifi which has a raft of access problems. There is no such thing as free broadband wireless (which is what they seem to be implying). My Blackberry costs $100 a month, for very slow Internet access. --Zeizmic 18:18, 6 January 2006 (UTC)
how does the cable car work?
- Depending what you mean by cable car, see the links on this page. smurrayinchester(User), (Talk) 16:54, 6 January 2006 (UTC)
Is the pen mightier than the keyboard and mouse?
Which one do you feel is better of the following. A touchscreen pen? or a mouse with keyboard? Which do you think is the easier, and which one do you prefer if given a choice?
- Personally, I'd go for the mouse and keyboard. Sum0 17:57, 6 January 2006 (UTC)
- Habit. I began using a keyboard in 1978. I didn't use a mouse until about 1992. So, to this day, I am more comfortable with a keyboard for 90% of my tasks and a mouse for simply moving windows around. If I were to have started with a mouse, I would probably use the mouse more. If I had started with a touchscreen pen, I'd probably use the touchscreen pen more. If I had started with a neural implant, I'd probably use the neural implant more. --Kainaw (talk) 19:30, 6 January 2006 (UTC)
- The touchscreen pen seems to have the disadvantage that the weight of your arm is not supported, as it is with a mouse. Thus, after hours of use, your arm will get tired. StuRat 20:47, 6 January 2006 (UTC)
- It depends on the application. For regular computing like editing Wikipedia, writing emails, browsing the internet, etc. I'm comfortable with a mouse and keyboard combo. But at my work a keyboard would just clutter things, so a touch screen is preferred. Dismas|(talk) 22:14, 6 January 2006 (UTC)
- I have a tablet PC... you can actually rest your arm on the screen with no problems, it's strong enough. For text entry, e.g. in my law lectures, I still find keyboard faster and more accurate. But in my maths lectures, I'll write with my stylus instead of writing on paper, because it's searchable. For other applications, I actually find that if there isn't much text entry (because handwriting is slow and inaccurate), using the stylus is far more natural, e.g. when casually browsing the net, or playing cards. enochlau (talk) 04:52, 7 January 2006 (UTC)
how to teach newborn to swim?
Why would you want to? 198.62.217.2 17:37, 6 January 2006 (UTC)
- This is actually common, 198. It can be a good idea for saftey reasons to introduce "swimming" at an early age. Read more here: [26] --Quasipalm 19:25, 6 January 2006 (UTC)
- My older sister almost drowned quite a few times as a toddler before she was finally taught to swim. It seems she simply didn't know any better and walked off piers or into the deep ends of pools. Since then me and my three other siblings were all taught to swim as infants. None of us had any near-drownings, and we all love the water. Seems like a good reason to me.
- As the the how, I've only seen it done in special classes. You usually first teach how to blow bubbles under water, and then when that comes naturally, you have one parent release the kid and another call them and they do the rest. — Laura Scudder ☎ 20:45, 6 January 2006 (UTC)
Los Angeles-class submarine mast pattern
Does anyone know the story behind the giraffe-like black-and-white pattern on the masts of this photograph of a surfaced Los Angeles-class submarine? (In case that link doesn't work, it's the sixth picture on this page. Sum0 17:56, 6 January 2006 (UTC)
- I can't find an answer either, but if I had to guess, it's probably camouflage for when the sub is running just under the surface with its masts extended. -- Cyrius|✎ 02:54, 7 January 2006 (UTC)
deepest part on the planet
what is the deepest part on the planet below sea level?
Finding Research Papers Online
I'm working on a project for science fair, and having some trouble tracking down the earlier research papers my sources cite. Google doesn't turn anything up, and I don't know many good search sites. How do professional scientist find papers? Black Carrot 18:37, 6 January 2006 (UTC)
- Try Google Scholar rather than just Google. Also, if you leave near a University or College, just pop in the library and ask what journals you should search. Most major universities have free access to journals as long as you're on a campus computer. Good luck. --Quasipalm 19:18, 6 January 2006 (UTC)
- Thanks. That's a start, but it doesn't have the papers I'm looking for, probably because they're a bit obscure and outside the mainstream. I'm using my project to test Rupert Sheldrake's experiments with 'the sense of being stared at'. Any other suggestions? Black Carrot 20:28, 6 January 2006 (UTC)
There is a psychology database similar to Medline and I think it includes parapsychology research. It will be available through most college or university libraries. Ask a librarian for help. The best starting point is often a paper that you do have, because the librarian can see how it is catalogued in the database and can then help you look for older but similar papers. There are a couple of American universities that have supported "paranormal" research, usually in association with the psychology dept, often under the name of parapsychology. You could call one of those depts and ask a secretary if a faculty member would be willing to talk to you once for an "interview to help with a school science project" and you might get lucky enough to get a few minutes of time. If so, ask their opinion of the research in that area and ask for suggestions on how to most efficiently find published research on the topic. They may be able to suggest specific journals, search terms or even authors to look for. Good luck. alteripse 20:53, 6 January 2006 (UTC)
You may also go directly to Rupert Sheldrake's books. He may describe his research there, or at least point you to more information in the notes. However, you may have trouble since often these sorts of pseudoscience authors take great care to hide their research from scrutiny. --Quasipalm 20:57, 6 January 2006 (UTC)
If your campus is subscribed, you'll also be able to use http://www.sciencedirect.com and http://www.engineeringvillage2.org. deeptrivia (talk) 23:35, 6 January 2006 (UTC)
You should check if your school or local library (etc.) subscribes to databases such as Thomson Gale Group or EBSCO host; These sites contain digital copies of articles from various scientific journals, some of which may be found as a hard copy in your library. If you live in a state such as Pennsylvania that has something similar to the AcessPA system, you can get access to these databases free with a library card. --Dragoon235 04:17, 7 January 2006 (UTC)
inventing a source of perpetual energy
How close are mankind from inventing a source of perpetual energy?
- As close as we always were. As far as anyone knows, it's prohibited by the laws of physics. -- SCZenz 18:41, 6 January 2006 (UTC)
- With one exception. The universe is believed to obey the law of conservation of energy. Therefore, the universe has a universal constant supply of energy. It never loses or gains any. It is just perpetually there. --Kainaw (talk) 19:27, 6 January 2006 (UTC)
- Though that doesnt stop people from trying. History of perpetual motion machines shows the various attempts at creating one. GeeJo (t) (c) • 19:37, 6 January 2006 (UTC)
Of course, there are many energy sources that will last for billions of years, so are as good as perpetual, like hydro, solar, tidal, wave, wind, and geothermal energy. And while each chunk of fuel for a nuclear reactor may only last a few years, there is enough nuclear fuel to power the world virtually forever. Renewable sources, like wood, are also good forever if properly managed. Only fossil fuels will be "used up" someday soon, perhaps decades or centuries, that's not certain. StuRat 20:40, 6 January 2006 (UTC)
- What if we were to figure out how to accelerate the transition from bio-waste into crude oil? Then, fossil fuels would also be a renewable energy source. We could keep pumping out those greenhouse gasses until we need a huge umbrella to cut down on solar heat. --Kainaw (talk) 20:46, 6 January 2006 (UTC)
- Well, you definitely can get methane from bacterial action on waste products, but I call this a "biofuel", not a "fossil fuel", to show it's source isn't "fossils". I don't know of any way to generate crude oil or coal from current waste products using bacteria, but I don't see why you would want to, as those forms both require refining and pollution controls while methane is ready to burn as is. StuRat 23:00, 6 January 2006 (UTC)
It's a bit more abstract, but the questioner might like to read Conservation of energy which states that energy is never created or destroyed, but is a constant. However, the Second law of thermodynamics states that the energy of an isolated system, while constant, is in a constant process of equalling out, meaning that the contained energy becomes more and more difficult to obtain in a general sense. --Quasipalm 20:52, 6 January 2006 (UTC)
- While energy can theoretically be changed into mass according to , this doesn't appear to happen anywhere on Earth. Energy seems to end up in the least usable form, which is heat. While usable energy can be generated from a heat differential, as in the case of geothermal energy, constant temperature heat is not a usable form of energy. StuRat 22:52, 6 January 2006 (UTC)
- Conversions from mass to energy do happen on Earth, but on a small scale; see Binding energy for example - in nuclear fusion/fission. enochlau (talk) 04:55, 7 January 2006 (UTC)
mining the moon
When mining the moon, what useful minerals would i find?
- According to Moon, you will find uranium, thorium, potassium, oxygen, silicon, magnesium, iron, titanium, calcium, aluminium and hydrogen. It doesn't state the quantities of each. --Kainaw (talk) 20:12, 6 January 2006 (UTC)
Also note that the cost of getting the minerals back to Earth would far exceed their value. StuRat 20:33, 6 January 2006 (UTC)
- You're assuming that they actually want to get it down to the Earth though. If they have a mining operation in place, then with just a bit more money (What's a few billion more?) they could refine the minerals there and start a sustainable colony. Dismas|(talk) 00:16, 7 January 2006 (UTC)
- Then they would need to get oxygen and water (not to mention a smelting plant) up to the colony on the moon, which would be just as prohibitively expensive. StuRat 00:45, 7 January 2006 (UTC)
- Supposedly the most valuable thing (probably the only economically exportable good) to mine on the Moon is Helium-3.--Pharos 05:09, 7 January 2006 (UTC)
Why does a pendulum work?
(no question)
- A mass at one end the pendulum is pulled downard by gravity. It accelerates, but it redirected by a pivot point so that the momentum is going back upward. Gravity then pulls it down again. This repeats. A pendulum will eventually stop due to air resistance, friction in the rotation joint, and so on. By adding energy (like the big weights in a grandfather clock), you can keep a pendulum going. For more, see pendulum. --Kainaw (talk) 20:44, 6 January 2006 (UTC)
- The characteristic of a pendulum which makes it ideal for time-keeping is that the period (amount of time for it to complete one full swing) is constant, even as the magnitude (distance of the full swing) reduces due to air resistance and friction. Thus, a pendulum can be used to measure time until it comes to a complete stop. StuRat 22:45, 6 January 2006 (UTC)
- I've always had trouble buying that. It seems like, if it takes a certain amount of time to get, say, from out horizontally to pointing at the floor (multiply by 4 for period), it will take less time to get from lower than horizontal to the floor. --Black Carrot 04:32, 7 January 2006 (UTC)
- The Black Carrot is right to be suspicious; it's not actually quite true. (See the pendulum article for a look at the math, but it's not simple.) However, it is a very good approximation. If the pendulum starts out horizontally, its full weight is acting perpendicular to the string and this sets it in motion very fast. If it starts out near the bottom of the swing, its weight is almost parallel to the string, so the force on it is a lot less, and so is its speed. As it works out, the period is not exactly the same in the two cases, but it's very close to the same (especially for small angles).
- Try it yourself. Take a ball of string and some tape. Unreel a few feet of string and tape the string to the ball where it comes off the ball. Tape the end of the string to the top of a doorframe. Set the ball to swinging just a couple of inches and time it for 10 swings back and forth. Then swing it up to a high angle, let it go, and time it again. I just did this and the times I got were between 19 and 20 seconds for 10 swings every time: my error in timing was probably larger than the difference between a long and a short swing. Of course, you will get different numbers according to how long you make the pendulum, but they will still agree with each other. (Because friction reduces the length of swing very fast with such a light pendulum, you might also try timing just 2 or 3 swings, but then the relative error of measurement is greater.)
- In order for it to be accurately true that the period is fixed, the pendulum would have to follow a cycloidal path rather than a circular one: see tautochrone curve. However, this turns out not to be useful for practical clockmaking, because the mechanism necessary to do that introduces too much friction. Instead, pendulum clocks were designed so that the drive mechanism would keep the angle of the swing relatively small, and as constant as possible. For the really accurate pendulum clocks that astronomers used before electronics superseded them, very long pendulums were were used (like say 10 feet), but they would swing a very short distance (just a few inches).
- --Anonymous, 05:45 UTC, January 7, 2006
Fooling credit score calculators
Is there any truth in Jaron Lanier's claim that people can and do arrange their finances in bizarre ways in order to get an improved credit score? ~~ N (t/c) 21:19, 6 January 2006 (UTC)
- The rules seem to be sufficiently bizarre that such opportunities would present themselves. For example, a similarly bizarre policy by airlines of charging more for a one-way trip than the corresponding round-trip led to the technique of buying a round-trip ticket and only using the first half. StuRat 02:04, 7 January 2006 (UTC)
Neural Net Image Categorization
Most of the information I can find on neural nets is either very basic and general, or owned by a company and unavailable to outsiders. Where can I find information on the construction of neural nets that leans towards the conceptual (I only know Java, and don't have time to decipher other languages) and towards a large number of inputs, say on the order of millions? Black Carrot 21:46, 6 January 2006 (UTC)
- I hope you realize such a neural net will require massive computing power to run at a reasonable speed. Also, what is the application ? Fluid dynamics ? StuRat 22:38, 6 January 2006 (UTC)
- I do realize that it will take some work to apply it the way I'm hoping to, but I don't think it's impossible, and I'm certainly willing to try. There are worse ways to waste time. I especially hope to find ways, as you mention, to reduce the number of inputs to a more manageable level, but I don't think I can go below the tens of thousands. I'm trying to find a way to search the web for actual images. Google is great, but it only does keyword searches, and I would like to be able to do more than that. I'd like something that can sepearate a set of pictures into a Yes pile and a No pile accurately, such as Tree v Not Tree. Naturally, neural nets lept to mind. The structures I've found so far, however, don't lend themselves well to this. It's not that they can't be set to sort into the right piles, it's that you have to have the piles sorted in the first place for backpropagation to work, and then the setup is fairly rigid, not dealing well with cases outside its specific expertise, and not dealing too well with new cases being added. The ideal search, though, would involve a progressive narrowing down, and would anticipate related cases. I've come up with some things I haven't seen anyone write about that I think would help, but I think things would go a lot faster if I could find out what the people who've been working on this for years have thought of. Any suggestions are appreciated. Black Carrot 01:04, 7 January 2006 (UTC)
- Wow, that's quite an ambitious project. It sounds like the problems you are having are quite similar to those for voice recognition software. Specifically, the software can recognize the difference between "circumvent" and "circumnavigate" if programmed specifically for that task and adjusted for a given voice, but does a poor job of identifying a random word in a random voice. Also, such software needs to "learn" differences between similar words, which requires a great deal of user input to "train" it. I'm not even sure how you would define which picture is a tree and which is not, considering cases like a tree and a person, a tree branch, a tree sprout, a bush, a flower, etc.
- I have thought of a much less ambitious search method, which could search for copies of an exact picture, possibly with a different scale. This could compare number of pixels of each color and look for a given ratio, as well as looking for colors to be in the same relative position on the pic. Complications such as mirror images, pics trimmed differently, non-uniform scaling, and different color balances would require quite a bit of coding to solve, but does seem doable. I've often found a pic via a web search which is just what I want as far as subject, but is too small. I would like a way to search for a full sized copy of the pic, even if the page doesn't contain the keywords I used in the initial picture search. Is that a project which would interest you ? StuRat 01:31, 7 January 2006 (UTC)
- 'Fraid not. I would expect that to be one of the functions of what I'm going for, but it's not by itself what I want. Besides, how many duplicate pictures are there floating around?
- You see the problem pretty clearly. What I think I have to go for is something that, in its structure, mimics the way we see things. Not that it mimics the brain, that would be a waste of time and I don't have the background anyway. Here's how I see it. The integer inputs(from 0-255 in Java), form a massively multidimensional graph, at each point of which the output(a real number, ideally either 0 or 1) can be represented by a color, either red or blue, with black or white at the boundaries. Makes a nice, manageable picture, except for the countless thousands of unimaginable axes. I think 'phase space' is the technical name for things like this. Now, the most basic form of neural net (sans sigmoid) will draw a beautiful diagonal gradient, which is useless to me. A single-layer network that makes use of the sigmoid function will have a straight line(plane, hyperplane, however many dimensions) between one clear area of blue and one clear area of red, at any slant and position you want. Good start. A two-layer one will draw as many of those as you want(with colors strengthening each other where they overlap), then shove all the outputs above a certain value to one color, and all the ones below it to another, and you have a shape on the graph, most any basic shape you want. Great. Add another layer, and you can have a bunch of shapes scattered across the graph, making it nice and flexible. Now, within those shapes the output will be 0(No), and beyond them it will be 1(Yes), or vice versa, and you can clearly see which pictures(which points) will be accepted and which will be rejected. Dandy. Except that that's not what the graph needs to look like to mimic the trends in the positions of actual images. It's hard to say exactly what it would look like, but I can get the concepts down and let the program take care of the rest. A few characteristics of the goal graph: area around image points, axis-parallel lines out from points, perpendicular rotation of shape about origin, image point shadows, threads between points, and quite a few others I haven't nailed down. I've solved the first one. The idea there is that, for any clear picture, there is some amount of error or static you can add to each pixel and still keep the picture essentially and recognizably what it is. On the graph, this means that there is a certain distance out you can go in any direction (direction=one color of one pixel in the image, or movement parallel to an axis) or combination of directions from the point that represents the clear image, and still be in essentially the same place. So, if that point is one color, the area around it must be as well, but not the area beyond that. It's possible to draw a nice simple square/box/hypercube around that area with a two-level bit of neural net, but with the number of sides needed(two per axis, >80000 axes), that box is prohibitively expensive in terms of how many nodes the net contains. I eventually worked out that if, instead of multiplying each input by a weight, then summing them and running them through the sigmoid, you add a bias to each input, square each result, then do all the rest, it draws a nice little resizable egg of whichever color around any point you want, with one layer, startlingly few nodes, and the added benefit that the list of the biases, laid out in a rectangle, is the clear picture you started with. So, that's where I'm coming from. Any suggestions? --Black Carrot 03:59, 7 January 2006 (UTC)
can normal people get atum bombe?
using household stuff
- No, not unless you keep enriched uranium and plutonium in the cupboard next to the enriched flour. If so, you need to be extra careful when baking bread. StuRat 00:02, 7 January 2006 (UTC)
January 7
Penis
Is it average for a 14 year old to have a 6 3/4 inch long penis and have 4 inches gurth?
- That depends on if you are looking at a naked 14 year old girl at the time. StuRat 00:10, 7 January 2006 (UTC)
- Studies on penis size are generally done on adults. So you probably will not find a study anywhere that gives averages for teenagers. Although, according to the Human penis size article, you're off to a good start. Dismas|(talk) 00:12, 7 January 2006 (UTC)
- I second the above. I'm very familiar with the research on human penis size, and there has never been any research on 14 year olds (for obvious reasons). That said, let me warn you that there has never been high-quality research done on human penis size, regardless of age - it's just not a practical undertaking. The best we can say, given the limits of our current research, is that the average American penis is between 5 and 7 inches. So you're doing fine. --George 05:18, 7 January 2006 (UTC)
Ethanol as fuel
If the U.S. capitalizes on the ethanol gas, how much do you think gas would go down? How much would Americans be saving?
- Cars can run up to about 25% ethanol with conventional engines, which would make some difference, but not too much, on a global scale, especially since 10% ethanol is already used in many areas. On the other hand, if car engines were retooled to use 99% ethanol (with 1% gasoline in a special tank for cold weather starting), then that would have quite a significant impact on world petroleum consumption and thus prices. Unfortunately, ethanol prices would likely skyrocket, at least until production caught up with demand. A mixture of technologies, like ethanol, diesel, electric, hydrogen fuel cell, and compressed natural gas, is likely to avoid the type of supply shocks we get when solely dependent on one type of fuel. For example, if a family owns a gasoline car and an ethanol car, they could switch which one they drive dependent on the relative prices of each. This price elasticity would cause price stability, unlike the current inelastic price curve for gasoline, which causes price instability. Flexible fuel vehicles are thus ideal for managing prices. StuRat 00:16, 7 January 2006 (UTC)
- Pay close attention to Brazil. They are in the middle of an attempted transition between petrol and ethanol. There are problems. Many of them appear to be solved. --Kainaw (talk) 00:35, 7 January 2006 (UTC)
- However, Brazil has warmer weather than the US, so doesn't need the same alternate cold-weather gasoline starting tank. StuRat 00:38, 7 January 2006 (UTC)
computer display screen readability
Hello,
What makes some handheld computer and cell phone displays easier to read than others, both indoors and outside? If i was looking at a specification for different types of displays, what attributes contribute to readability the most?
thank you so much for answering my question!
--Linda
- A backlight is very important, as is the text size, those should be in the specs. However, the contrast of the display relative to the background and the reflectivity and hardness of the glass or plastic bezel (and thus the resistance to scratches) is not something you are likely to see in the specs, so you should check each one out in person, if possible. One warning, don't fall for the stupid plastic film that "shows you" what it will look like when running, actually test it out. StuRat 00:26, 7 January 2006 (UTC)
internet? problem
hey i am really having a hard time. can you give me a website to find background information on linear dynamics? i've been working on it for 10 hours and can't find anything about background stuff. grateful for all help. --sami
- Hi Sami. Have you seen Dynamical system#Linear dynamical systems already? --Quasipalm 03:55, 7 January 2006 (UTC)


![{\displaystyle \left[{\frac {1}{R+j\omega L}}+{\frac {1}{j\omega C}}\right]^{-1}\;}](/media/api/rest_v1/media/math/render/svg/af37196298b868f3ba464f9d3bb41648d8d67f27)
![{\displaystyle \left[{\frac {1}{R+j\omega L}}+{j\omega C}\right]^{-1}\;}](/media/api/rest_v1/media/math/render/svg/81e976510050909d00a4911b31e6dfa651b91353)





