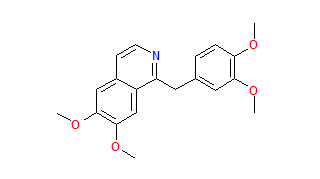
Papaverine
| 1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-isoquinoline[1] | |
| CAS number 58-74-2,[2] |
ATC code G04BE02, A03AD01 |
| Chemical formula | C20H21NO4[1] |
| Molecular weight | 339.385 g/mol[1] |
| Bioavailability | 80%[3] |
| Metabolism | Hepatic[3] |
| Elimination half-life | 1.5-2 hours[3] |
| Excretion | Renal[3] |
| Pregnancy category | USA: C[4] |
| Legal status | ? |
| Routes of administration | Oral, intravenous, intramuscular, rectal,[5] intracavernosal |
Papaverine is an opium alkaloid used primarily in the treatment of visceral spasm, vasospasm (especially those involving the heart and the brain), and occasionally in the treatment of erectile dysfunction[3]. While it is found in the opium poppy, papaverine differs in both structure and pharmacological action from the other opium alkaloids.
Uses
Papaverine is approved to treat spasms of the gastointestinal tract, bile ducts and ureter and for use as a cerebral and coronary vasodilator[3] in subarachnoid hemorrhage (combined with balloon angioplasty)[6] and coronary artery bypass surgery[7]. Papaverine may also be used as a smooth muscle relaxant in microsurgery where it applied directly to blood vessels.
The in vivo mechanism of action is not entirely clear, but an inhibition of the enzyme phosphodiesterase causing elevation of cyclicAMP levels is significant. It may also alter mitochondrial respiration.
Side effects
Frequent side effects of papaverine treatment include polymorphic ventricular tachycardia, constipation, interference with sulphobromophthalein[8] retention test (used to determine hepatic function), increased transaminase levels, increased alkaline phosphatase levels, hyperbilirubinemia, somnolence, and vertigo[3].
Rare side effects include flushing of the face, hyperhidrosis (excessive sweating), cutaneous eruption, arterial hypotension, tachycardia, lack of appetite, jaundice, eosinophilia, thrombopenia, mixed hepatitis, headache, allergic reaction, chronic active hepatitis,[3] and paradoxical aggravation of cerebral vasospasm[9].
Formulations and Tradenames
Papaverine is available as a conjugate of hydrochloride, codecarboxylate, adenylate, and teprosylate[10]. It was also once available as a salt of hydrobromide, camsylate, cromesilate, nicotinate, and phenylglycolate. The hydrochloride salt is available for intramuscular, intravenous, rectal and oral administration.[5] The teprosylate is available in intravenous, intramuscular, and orally administered formulations[11]. The codecarboxylate is available in oral form, only,[12] as is the adenylate[13].
The codecarboxylate is sold under the name Albatran®,[14] the adenylate as Dicertan®,[15] and the hydrochloride salt is sold variously as Artegodan® (Germany), Cardioverina® (countries outside Europe and the United States), Dispamil® (countries outside Europe and the United States), Opdensit® (Germany), Panergon® (Germany), Paverina Houde® (Italy, Belgium), Pavacap (United States), Pavadyl® (United States), Papaverin-Hamelin® (Germany), Paveron® (Germany), Spasmo-Nit® (Germany),[5] Cardiospan®, Papaversan®, Cepaverin®, Cerespan®, Drapavel®, Forpaven®, Papalease®, Pavatest®, Paverolan®, Therapav® (France[16]), Vasospan®, Cerebid®, Delapav®, Dilaves®, Durapav®, Dynovas®, Optenyl®, Pameion®, Papacon®, Pavabid®, Pavacen®, Pavakey®, Pavased®, Pavnell®, Alapav®, Myobid®, Vasal®, Pamelon®, Pavadel®, Pavagen®, Ro-Papav®, Vaso-Pav®, Papanerin-hcl®, Qua bid®, Papital T.R.®, Paptial T.R.®, Pap-Kaps-150®.[17]
References
- a b c "SID 544606 -- PubChem Substance Summary". Retrieved 25 September.
{{cite web}}: Check date values in:|accessdate=(help); Unknown parameter|accessyear=ignored (|access-date=suggested) (help) National Center for Biotechnology Information. - a "Papaverine Material Safety Data Sheet". Retrieved 25 September.
{{cite web}}: Check date values in:|accessdate=(help); Unknown parameter|accessyear=ignored (|access-date=suggested) (help) - a b c d e f g h Unknown (2000). "PAPAVERINE". Molécule(s) de base : PAPAVERINE. Biam. Retrieved 25 September.
{{cite web}}: Check date values in:|accessdate=(help); Cite has empty unknown parameter:|1=(help); Unknown parameter|accessyear=ignored (|access-date=suggested) (help) (French) - a Unknown (2004). "Who should not take papaverine?". papaverine Consumer Drug Information. Cerner Multum, Inc. Retrieved 26 September.
{{cite web}}: Check date values in:|accessdate=(help); Unknown parameter|accessyear=ignored (|access-date=suggested) (help) - a b c Unknown (1999). "PAPAVERINE CHLORHYDRATE". Molécule(s) de base : PAPAVERINE. Biam. Retrieved 25 September.
{{cite web}}: Check date values in:|accessdate=(help); Unknown parameter|accessyear=ignored (|access-date=suggested) (help) (French) - a Liu, James K.; Couldwell, William T (2005). "Intra-arterial papaverine infusions for the treatment of cerebral vasospasm induced by aneurysmal subarachnoid hemorrhage". Neurocritical Care. 2 (2): 124–32. PMID 16159054.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Fulltext options List of Library Holdings - a Takeuchi K, Sakamoto S, Nagayoshi Y, Nishizawa H, Matsubara J (2004). "Reactivity of the human internal thoracic artery to vasodilators in coronary artery bypass grafting". European Journal of Cardio-Thoracic Surgery. 26 (5): 956–9. PMID 15519189.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Fulltext options List of Library Holdings - a "SID 149219 -- PubChem Substance Summary". Retrieved 26 September.
{{cite web}}: Check date values in:|accessdate=(help); Unknown parameter|accessyear=ignored (|access-date=suggested) (help) National Center for Biotechnology Information. - a Clyde BL, Firlik AD, Kaufmann AM, Spearman MP, Yonas H (1996). "Paradoxical aggravation of vasospasm with papaverine infusion following aneurysmal subarachnoid hemorrhage. Case report". Journal of Neurosurgery. 84 (4): 690–5.
{{cite journal}}: CS1 maint: multiple names: authors list (link) PMID 8613866 - a "Molécule de base : PAPAVERINE". Retrieved 26 September.
{{cite web}}: Check date values in:|accessdate=(help); Unknown parameter|accessyear=ignored (|access-date=suggested) (help) Biam. - a Unknown (1999). "PAPAVERINE TEPROSILATE". Molécule(s) de base : PAPAVERINE. Biam. Retrieved 26 September.
{{cite web}}: Check date values in:|accessdate=(help); Unknown parameter|accessyear=ignored (|access-date=suggested) (help) (French) - a Unknown (1998). "PAPAVERINE CODECARBOXYLATE". Molécule(s) de base : PAPAVERINE. Biam. Retrieved 26 September.
{{cite web}}: Check date values in:|accessdate=(help); Unknown parameter|accessyear=ignored (|access-date=suggested) (help) (French) - a Unknown (1998). "PAPAVERINE ADENYLATE". Molécule(s) de base : PAPAVERINE. Biam. Retrieved 26 September.
{{cite web}}: Check date values in:|accessdate=(help); Unknown parameter|accessyear=ignored (|access-date=suggested) (help) (French) - a "SID 660773 PubChem Substance Summary". Retrieved 25 September.
{{cite web}}: Check date values in:|accessdate=(help); Unknown parameter|accessyear=ignored (|access-date=suggested) (help) National Center for Biotechnology Information. - a "SID 660767 -- PubChem Substance Summary". Retrieved 25 September.
{{cite web}}: Check date values in:|accessdate=(help); Unknown parameter|accessyear=ignored (|access-date=suggested) (help) National Center for Biotechnology Information. - a "THERAPAV (PRODUIT PUR) - Détail". Retrieved 26 September.
{{cite web}}: Check date values in:|accessdate=(help); Unknown parameter|accessyear=ignored (|access-date=suggested) (help) CSST - Service du répertoire toxicologique. (French) - a "SID 660767 -- PubChem Substance Summary - Depositor-Supplied Synonyms: All". Retrieved 26 September.
{{cite web}}: Check date values in:|accessdate=(help); Unknown parameter|accessyear=ignored (|access-date=suggested) (help) National Center for Biotechnology Information.