Pummerer rearrangement
The Pummerer rearrangement is an organic reaction whereby an alkyl sulfoxide rearranges to an α-acyloxy-thioether in the presence of acetic anhydride.[1] [2] In this reaction, sulfur is reduced while adjacent carbon is oxidized.
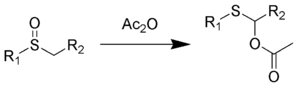
Several reviews have been published.[3] [4] [5]
The usage of α-acyl sulfoxides and Lewis acids, such as TiCl4 and SnCl4, allow the reaction to proceed at lower temperatures (0 °C).[6]
Mechanism
The mechanism of the Pummerer rearrangement begins with the acylation of the sulfoxide (1 and 2). Compound 3 undergoes elimation to produce the sulfonium ion 4. Acetate adds to the sulfonium ion to give the final product 5.
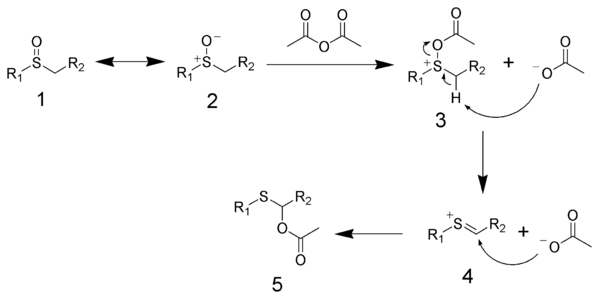
Other anhydrides and acyl halides can give similar products. Inorganic acids can also give this reaction.This product can be converted to aldehyde or ketone by hydrolysis.[7]
Variations
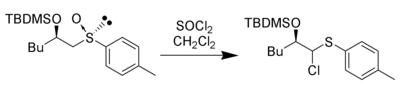
The sulfonium ion can be trapped by both intramolecular and intermolecular nucleophiles forming carbon-carbon bonds and carbon-heteroatom bonds. For example, thionyl chloride can be used to generate and trap the sulfonium ion:[8]
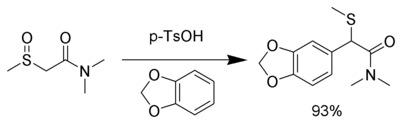
Likewise, nucleophiles (such as veratrole [9]) can also be used.
Pummerer fragmentation
When the α-organic residue can form a very good leaving group this group and not the α-hydrogen atom will eliminate in the intermediate step in a Pummerer fragmentation [10]. This reaction type is demonstrated below with a set of sulfoxides and the anhydride of trifluoroacetic acid (TFAA):
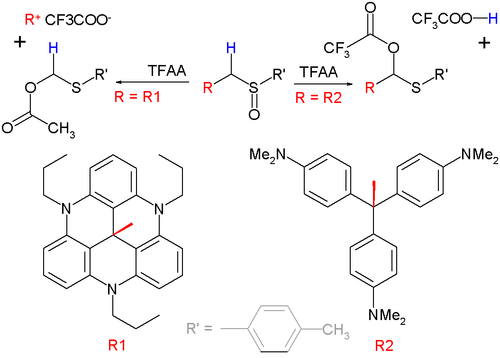
The organic group on the right is the moderately methyl violet carbocation with pKR+ = 9.4 leading to a classical Pummerer rearrangement. The reaction on the left is a fragmentation because the leaving group with pKR+ = 9.4 is particularly stable.
See also
References
- ^ Pummerer, R. Ber. 1909, 42, 2282.
- ^ Pummerer, R. Ber. 1910, 43, 1401.
- ^ De Lucchi, O.; Miotti, U.; Modena, G. Org. React. 1991, 40, 157-184. (Review)
- ^ Padwa, A.; Gunn, D. E., Jr.; Osterhout, M. H. Synthesis 1997, 1353-1377. (Review)
- ^ Padwa, A.; Rur, S. K.; Danca, M. d.; Ginn, J. D.; Lynch, S. M. Synlett 2002, 851-862. (Review)
- ^ Stamos, I. K. Tetrahedron Lett. 1986, 27, 6261.
- ^ Meffre, P.; Durand, P.; Le Goffic, F. Organic Syntheses, Coll. Vol. 10, p.562 (2004); Vol. 76, p.123 (1999). (Article)
- ^ Kosugi, H.; Watanabe, Y.; Uda, H. Chem. Lett. 1989, 1865.
- ^ Ishibashi, H. et al. Chem. Pharm. Bull. 1989, 37, 3396.
- ^ Pummerer fragmentation vs. Pummerer rearrangement: a mechanistic analysis Benoît Laleu, Marco Santarem Machado and Jérôme Lacour Chem. Commun., 2006, 2786 - 2788, doi:10.1039/b605187a
