Diketene
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
4-methylideneoxetan-2-one
| |||
| Other names
γ-methylenebutyrolactone
| |||
| Identifiers | |||
| ECHA InfoCard | 100.010.562 | ||
CompTox Dashboard (EPA)
|
|||
| Properties | |||
| C4H4O2 | |||
| Molar mass | 84.08 g mol−1 | ||
| Density | 1.09 g cm−3 | ||
| Melting point | −7 °C | ||
| Boiling point | 127 °C | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Diketene is an organic compound formed by dimerization of ketene. Diketene is a member of the oxetane family. It is used as a chemical reagent in organic chemistry[1]. It is a colorless liquid and heating regenerates the ketene monomer. Alkylated ketenes also dimerize with ease and form substituted diketenes.
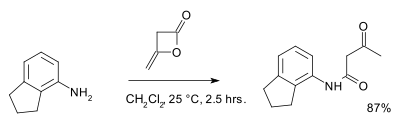
Diketene readily hydrolyses in water forming acetoacetic acid, its half life in pure water is approximately 45 minutes a 25°C at 2<pH<7.[2] Diketene also reacts with alcohols and amines to the corresponding acetoacetic acid derivatives. An example is the reaction with 2-aminoindane:[3]
Despite its high reactivity as an alkylating agent, and unlike analogue β-lactones propiolactone and β-butyrolactone, diketene is inactive as a carcinogen, possibly due to the instability of its DNA adducts.[4]
Certain diketenes with two aliphatic chains are used industrially to improve hydrophobicity in paper.
References
- ^ Beilstein E III/IV 17: 4297
- ^ Rafael Gómez-Bombarelli, Marina González-Pérez, María Teresa Pérez-Prior, José A. Manso, Emilio Calle and Julio Casado (2008). "Kinetic Study of the Neutral and Base Hydrolysis of Diketene". Phys. Org. Chem: n/a. doi:10.1002/poc.1483.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kiran Kumar Solingapuram Sai, Thomas M. Gilbert, and Douglas A. Klumpp (2007). "Knorr Cyclizations and Distonic Superelectrophiles". J. Org. Chem. 72: 9761–9764. doi:10.1021/jo7013092.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rafael Gómez-Bombarelli, Marina González-Pérez, María Teresa Pérez-Prior, José A. Manso, Emilio Calle and Julio Casado (2008). "Chemical Reactivity and Biological Activity of Diketene". Chem. Res. Toxicol. 21: 1964–1969. doi:10.1021/tx800153j.
{{cite journal}}: CS1 maint: multiple names: authors list (link)