Bicalutamide
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | Bicalutamide: • /ˌbaɪkəˈluːtəmaɪd/[1] • BY-kə-LOO-tə-myde[1] |
| Trade names | Casodex, Calutex, others |
| Other names | ICI-176,334; ZD-176,334 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697047 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth[2] |
| Drug class | Nonsteroidal antiandrogen |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Well-absorbed; absolute bioavailability unknown[3] |
| Protein binding | Racemate: 96.1%[2] (R)-Isomer: 99.6%[2] (Mainly to albumin)[2] |
| Metabolism | Liver (extensively):[4][9] • Hydroxylation (CYP3A4) • Glucuronidation (UGT1A9) |
| Metabolites | • Bicalutamide glucuronide • Hydroxybicalutamide • Hydroxybicalutamide gluc. (All inactive)[4][2][5][6] |
| Elimination half-life | Single-dose: 5.8 days[7] Continuous: 7–10 days[8] |
| Excretion | Feces: 43%[4] Urine: 34%[4] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.126.100 |
| Chemical and physical data | |
| Formula | C18H14F4N2O4S |
| Molar mass | 430.37 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture (of (R)- and (S)-enantiomers) |
| Melting point | 191 to 193 °C (376 to 379 °F) (experimental) |
| Boiling point | 650 °C (1,202 °F) (predicted) |
| Solubility in water | 0.005 |
| |
| |
| (verify) | |
Bicalutamide, sold under the brand name Casodex among others, is an antiandrogen medication that is primarily used to treat prostate cancer.[10] It is typically used together with a gonadotropin-releasing hormone (GnRH) analogue or surgical removal of the testicles to treat metastatic prostate cancer (mPC).[11][10][12] To a lesser extent, it is used at high doses for locally advanced prostate cancer (LAPC) as a monotherapy without castration.[4][2][13] Bicalutamide was also previously used as monotherapy to treat localized prostate cancer (LPC), but authorization for this use was withdrawn following unfavorable trial findings.[13][14][15][16] Besides prostate cancer, bicalutamide is limitedly used in the treatment of excessive hair growth and scalp hair loss in women,[17][18] as a puberty blocker and component of feminizing hormone therapy for transgender girls and women,[19] to treat gonadotropin-independent early puberty in boys,[20] and to prevent overly long-lasting erections in men.[21] It is taken by mouth.[10]
Common side effects of bicalutamide in men include breast growth, breast tenderness, and hot flashes.[10] Other side effects in men include feminization and sexual dysfunction.[22][23] Some side effects like breast changes and feminization are minimal when combined with castration.[24] While the medication appears to produce few side effects in women, its use in women is not explicitly approved by the Food and Drug Administration (FDA) at this time.[25][10] Use during pregnancy may harm the baby.[10] In men with early prostate cancer, bicalutamide monotherapy has been found to increase the likelihood of death from causes other than prostate cancer.[26][13] Bicalutamide produces abnormal liver changes necessitating discontinuation in around 1% of people.[27][13] Rarely, it has been associated with cases of serious liver damage,[10] serious lung toxicity,[3] and sensitivity to light.[28][29] Although the risk of adverse liver changes is small, monitoring of liver function is recommended during treatment.[10]
Bicalutamide is a member of the nonsteroidal antiandrogen (NSAA) group of medications.[3] It works by selectively blocking the androgen receptor (AR), the biological target of the androgen sex hormones testosterone and dihydrotestosterone (DHT).[30] It does not lower androgen levels.[3] The medication can have some estrogen-like effects in men when used as a monotherapy due to increased estradiol levels.[31][32][33] Bicalutamide is well-absorbed, and its absorption is not affected by food.[2] The elimination half-life of the medication is around one week.[2][10] It shows peripheral selectivity in animals, but crosses the blood–brain barrier and affects both the body and brain in humans.[2][34]
Bicalutamide was patented in 1982 and approved for medical use in 1995.[35] It is on the World Health Organization's List of Essential Medicines.[36] Bicalutamide is available as a generic medication.[37] The drug is sold in more than 80 countries, including most developed countries.[38][39][40] It was at one time the most widely used antiandrogen in the treatment of prostate cancer, with millions of men with the disease having been prescribed it.[23][41][42][43][44] Although bicalutamide is also used for other indications besides prostate cancer, the vast majority of prescriptions appear to be for treatment of prostate cancer.[44]
Medical uses
[edit]Bicalutamide is approved for and mainly used in the following indications:[45]
- Metastatic prostate cancer (mPC) in men in combination with a gonadotropin-releasing hormone (GnRH) analogue or surgical castration at 50 mg/day[27][4][46]
- Locally advanced prostate cancer (LAPC) in men as a monotherapy at 150 mg/day (not approved for this use in the United States)[4][2][13][47]
In Japan, bicalutamide is uniquely used at a dosage of 80 mg/day both in combination with castration and as a monotherapy in the treatment of prostate cancer.[48][49]
Bicalutamide is also employed for the following off-label (non-approved) indications:
- To reduce the effects of the testosterone flare at the initiation of GnRH agonist therapy in men[50][51]
- Androgen-dependent skin and hair conditions such as acne, seborrhea, excessive hair growth, and scalp hair loss in women as well as high testosterone levels due to polycystic ovary syndrome (PCOS) in women, at 25 to 50 mg/day generally in combination with a birth control pill[17][52][53][54][55][56][57]
- Feminizing hormone therapy for transgender women in combination with an estrogen usually at 50 mg/day[19][58][59][60][61][62][63]
- Peripheral precocious puberty in boys at 12.5 to 100 mg/day in combination with an aromatase inhibitor like anastrozole, especially for familial male-limited precocious puberty (testotoxicosis)[27][64][65][66][67][68][20][69]
- Overly long-lasting erections in men at 50 mg per week to 50 mg every other day[70][71][72][73][3][7][21]
The medication has been suggested for but has uncertain effectiveness in the following indication:
- Hypersexuality and paraphilias, particularly in combination with chemical castration[74][75][76][77][78][79]
For more information on these uses, see the medical uses of bicalutamide article.
Available forms
[edit]Bicalutamide is available for the treatment of prostate cancer in most developed countries,[80][38][81] including over 80 countries worldwide.[39][40] It is available in 50 mg, 80 mg (in Japan),[48] and 150 mg tablets for oral administration.[82][83] The drug is registered for use as a 150 mg/day monotherapy for the treatment of LAPC in at least 55 countries,[2] with the U.S. being a notable exception where it is registered only for use at a dosage of 50 mg/day in combination with castration.[84] No other formulations or routes of administration are available or used.[82] All formulations of bicalutamide are specifically indicated for the treatment of prostate cancer alone or in combination with surgical or medication castration.[4] Due to the low water solubility of bicalutamide, bicalutamide in oral bicalutamide tablets is micronized to ensure small and consistent particle sizes and optimize oral bioavailability.[85][2]
A combined formulation of bicalutamide and the GnRH agonist goserelin in which goserelin is provided as a subcutaneous implant for injection and bicalutamide is included as 50 mg tablets for oral ingestion is marketed in Australia and New Zealand under the brand name ZolaCos CP (Zoladex–Cosudex Combination Pack).[81][86][87][88]
Contraindications
[edit]Bicalutamide is pregnancy category X, or "contraindicated in pregnancy", in the U.S.,[27] and pregnancy category D, the second most restricted rating, in Australia.[89] As such, it is contraindicated in women during pregnancy, and women who are sexually active and who can or may become pregnant are strongly recommended to take bicalutamide only in combination with adequate contraception.[90][91] It is unknown whether bicalutamide is excreted in breast milk, but many drugs are excreted in breast milk, and for this reason, bicalutamide treatment is similarly not recommended while breastfeeding.[3][27]
In individuals with severe, though not mild-to-moderate hepatic impairment, there is evidence that the elimination of bicalutamide is slowed, and hence, caution may be warranted in these patients as circulating levels of bicalutamide may be increased.[2][92] In severe hepatic impairment, the elimination half-life of the active (R)-enantiomer of bicalutamide is increased by about 1.75-fold (76% increase; elimination half-life of 5.9 and 10.4 days for normal and impaired patients, respectively).[13][93][94] The elimination half-life of bicalutamide is unchanged in renal impairment.[84]
Side effects
[edit]| Frequency | Class of effect | Effect |
|---|---|---|
| Very common (≥10%) | Reproductive system and breast disorders | • Breast tenderness[a] • Gynecomastia[a] |
| Common (1–10%) | General and psychiatric disorders | • Asthenia • Decreased libido • Erectile dysfunction • Hot flashes |
| Skin and subcutaneous tissue disorders |
• Decreased body hair | |
| Hepato-biliary disorders | • Elevated liver enzymes[b] | |
| Uncommon (0.1–1%) | Immune system disorders and hypersensitivity reactions | • Angioedema • Hives |
| Rare (<0.1%) or unknown | Respiratory disorders | • Lung disease[c] |
| Skin and subcutaneous tissue disorders | • Sensitivity to light | |
| Hepato-biliary disorders | • Liver toxicity[c] | |
| ||
The side effect profile of bicalutamide is highly dependent on sex; that is, on whether the person is male or female. In men, due to androgen deprivation, a variety of side effects of varying severity may occur during bicalutamide treatment, with breast pain/tenderness and gynecomastia (breast development/enlargement) being the most common.[102][103] Gynecomastia occurs in up to 80% of men treated with bicalutamide monotherapy, and is of mild-to-moderate severity in more than 90% of affected men.[103][104] In addition to breast changes, physical feminization and demasculinization in general, including reduced body hair growth, decreased muscle mass and strength, feminine changes in fat mass and distribution, reduced penile length, and decreased semen/ejaculate volume, may occur in men.[102][105][22][106] Other side effects that have been observed in men and that are similarly related to androgen deprivation include hot flashes, sexual dysfunction (e.g., loss of libido, erectile dysfunction), depression, fatigue, weakness, and anemia.[102][107][108] However, most men have preserved sexual function with bicalutamide monotherapy.[109][110] In females, due to the minimal biological importance of androgens in this sex,[111][112] the side effects of pure antiandrogens or NSAAs are few, and bicalutamide has been found to be very well tolerated.[25] However, bicalutamide has been found to increase levels of total and LDL cholesterol in women.[113][114][57] The non-pharmacological side-effect profile of bicalutamide (i.e., side effects not related to its antiandrogenic activity) is said to be similar to that with placebo.[115] In any case, general side effects of bicalutamide that might occur in either sex include diarrhea, constipation, abdominal pain, nausea, dry skin, itching, and rash.[107][3][116][117][118][119] The drug is well-tolerated at higher dosages than 50 mg/day, up to 600 mg/day, with rare additional side effects.[84][120][121]
Bicalutamide has been associated with abnormal liver function tests such as elevated liver enzymes.[107][13] In the Early Prostate Cancer (EPC) clinical programme of bicalutamide for LPC and LAPC, the rate of abnormal liver function tests with bicalutamide monotherapy was 3.4% relative to 1.9% for placebo.[13][122] However, higher rates, up to 11%, have been seen in other studies.[18][27] Hepatic changes that have necessitated discontinuation of bicalutamide, such as marked increases in liver enzymes or hepatitis, have occurred in 0.3 to 1.5% of men in clinical trials, or approximately 1% overall.[27][13][33][122][123] Elevated liver enzymes with bicalutamide usually occur within the first 3 to 6 months of treatment.[107][27] Monitoring of liver function during treatment is recommended, particularly in the first few months.[13][102] In men with early prostate cancer, bicalutamide monotherapy has been found to increase non-prostate cancer mortality.[26][124][13] The reasons for the increase in mortality with bicalutamide in these men are unknown, but possible factors could include androgen deprivation or drug-related toxicity of bicalutamide.[125][126]
There are 10 published case reports of liver toxicity associated with bicalutamide as of 2022.[127][128][129][130] Death occurred in 2 of these cases.[127][131][132] Hundreds of additional cases of liver complications in people taking bicalutamide exist in the FDA Adverse Event Reporting System (FAERS) database.[133] In all of the published case reports of liver toxicity with bicalutamide, the onset of symptoms was within the first 6 months of treatment.[128][129][130] Symptoms that may indicate liver dysfunction include nausea, vomiting, abdominal pain, fatigue, anorexia, "flu-like" symptoms, dark urine, and jaundice.[27] There are also published case reports of interstitial pneumonitis and eosinophilic lung disease associated with bicalutamide.[134][135][136] along with hundreds of additional instances in the FAERS database as well.[133] Interstitial pneumonitis can potentially progress to pulmonary fibrosis and may be fatal. Symptoms that may indicate lung dysfunction include dyspnea (difficult breathing or shortness of breath), cough, and pharyngitis (inflammation of the pharynx, resulting in sore throat).[137] The exact incidence of liver toxicity and interstitial pneumonitis with bicalutamide are unknown, but both are said to be very rare events.[129][138][139] A few cases of photosensitivity have been reported with bicalutamide.[28] Hypersensitivity reactions (drug allergy) like angioedema and hives have also uncommonly been reported in association with bicalutamide.[27]
Because it is an antiandrogen, bicalutamide has a theoretical risk of birth defects like ambiguous genitalia in male fetuses.[90][91][140][141] Due to its teratogenic capacity, contraception should be used in women taking bicalutamide who are fertile and sexually active.[142]
Comparison
[edit]The side effect profile of bicalutamide in men and women differs from that of other antiandrogens and is considered favorable in comparison.[143][110][144][145] Relative to GnRH analogues and the steroidal antiandrogen (SAA) cyproterone acetate (CPA), bicalutamide monotherapy has a much lower incidence and severity of hot flashes and sexual dysfunction.[109][110][104][146] In addition, unlike GnRH analogues and CPA, bicalutamide monotherapy is not associated with decreased bone mineral density or osteoporosis.[104][110] Conversely, bicalutamide monotherapy is associated with much higher rates of breast tenderness, gynecomastia, and feminization in men than GnRH analogues and CPA.[104] However, gynecomastia with bicalutamide is rarely severe and discontinuation rates due to this side effect are fairly low.[104][110] These differences in side effects between bicalutamide monotherapy, GnRH analogues, and CPA are attributed to the fact that whereas GnRH analogues and CPA suppress estrogen production, bicalutamide monotherapy does not lower estrogen levels and in fact actually increases them.[104]
Bicalutamide does not share the risk of neuropsychiatric side effects like fatigue as well as cardiovascular side effects like coagulation changes, blood clots, fluid retention, ischemic cardiomyopathy, and adverse serum lipid changes that CPA has been associated with.[146][147][148][149] It has a much lower risk of hepatotoxicity than flutamide and CPA and of interstitial pneumonitis than nilutamide.[150][110][131][151][152][153] The drug also does not share the unique risks of diarrhea with flutamide and nausea, vomiting, visual disturbances, and alcohol intolerance with nilutamide.[110][146][151] Unlike enzalutamide, bicalutamide is not associated with seizures or related central side effects like anxiety and insomnia.[154][155] However, although the risk of adverse liver changes with bicalutamide is low, enzalutamide differs from bicalutamide in having no known risk of elevated liver enzymes or hepatotoxicity.[156][157] In contrast to the SAA spironolactone, bicalutamide does not have antimineralocorticoid effects,[158] and hence is not associated with hyperkalemia, urinary frequency, dehydration, hypotension, or other related side effects.[159][160][161][146] In women, unlike CPA and spironolactone, bicalutamide does not produce menstrual irregularity or amenorrhea and does not interfere with ovulation or fertility.[52][162]
Overdose
[edit]A single oral dose of bicalutamide in humans that results in symptoms of overdose or that is considered to be life-threatening has not been established.[27][163] Dosages of up to 600 mg/day have been well tolerated in clinical trials,[164] and it is notable that there is a saturation of absorption with bicalutamide such that circulating levels of its active (R)-enantiomer do not further increase above a dosage of 300 mg/day.[2][164] Overdose is considered unlikely to be life-threatening with bicalutamide or other first-generation NSAAs (i.e., flutamide and nilutamide).[165] A massive overdose of nilutamide (13 grams, or 43 times the normal maximum 300 mg/day clinical dosage) in a 79-year-old man was uneventful, producing no clinical signs, symptoms, or toxicity.[166] There is no specific antidote for bicalutamide or NSAA overdose, and treatment should be based on symptoms, if any are present.[27][163]
Interactions
[edit]Bicalutamide is almost exclusively metabolized by CYP3A4.[4] As such, its levels in the body may be altered by inhibitors and inducers of CYP3A4.[7] (For a list of CYP3A4 inhibitors and inducers, see here.) However, in spite of the fact bicalutamide is metabolized by CYP3A4, there is no evidence of clinically significant drug interactions when bicalutamide at a dosage of 150 mg/day or less is co-administered with drugs that inhibit or induce cytochrome P450 enzyme activity.[13]
In-vitro studies suggest that bicalutamide may be able to inhibit CYP3A4 and, to a lesser extent, CYP2C9, CYP2C19, and CYP2D6.[2] Conversely, animal studies suggest that bicalutamide may induce cytochrome P450 enzymes.[2] In a clinical study, bicalutamide co-administered with the CYP3A4 substrate midazolam caused only a small and statistically non-significant increase in midazolam levels (+27%) presumably due to CYP3A4 inhibition.[2] However, this was well below increases in midazolam exposure with potent CYP3A4 inhibitors like ketoconazole (+1500%), itraconazole (+1000%), and erythromycin (+350%), and is considered to not be clinically important.[2] There is no indication of clinically significant enzyme inhibition or induction with bicalutamide at doses of 150 mg/day or below.[2]
Because bicalutamide circulates at relatively high concentrations and is highly protein-bound, it has the potential to displace other highly protein-bound drugs like warfarin, phenytoin, theophylline, and aspirin from plasma binding proteins.[103][107] This could, in turn, result in increased free concentrations of such drugs and increased effects and/or side effects, potentially necessitating dosage adjustments.[103] Bicalutamide has specifically been found to displace coumarin anticoagulants like warfarin from their plasma binding proteins (namely albumin) in vitro, potentially resulting in an increased anticoagulant effect, and for this reason, close monitoring of prothrombin time and dosage adjustment as necessary is recommended when bicalutamide is used in combination with these drugs.[167][168][169] However, in spite of this, no conclusive evidence of an interaction between bicalutamide and other drugs was found in clinical trials of nearly 3,000 patients.[107]
Pharmacology
[edit]Pharmacodynamics
[edit]Antiandrogenic activity
[edit]Bicalutamide acts as a highly selective competitive silent antagonist of the AR (IC50 = 159–243 nM), the major biological target of the androgen sex hormones testosterone and DHT, and hence is an antiandrogen.[30][170][171][172] The activity of bicalutamide lies in the (R)-isomer.[173] Due to its selectivity for the AR, bicalutamide does not interact importantly with other steroid hormone receptors and hence has no clinically relevant off-target hormonal activity (e.g., progestogenic, estrogenic, glucocorticoid, antimineralocorticoid).[174][34][173][45] However, it has been reported that bicalutamide has weak affinity for the progesterone receptor (PR), where it is an antagonist, and hence it could have some antiprogestogenic activity.[175] Bicalutamide does not inhibit 5α-reductase nor is known to inhibit other enzymes involved in androgen steroidogenesis (e.g., CYP17A1).[176] Although it does not bind to the estrogen receptors (ERs), bicalutamide can increase estrogen levels secondarily to AR blockade when used as a monotherapy in males, and hence can have some indirect estrogenic effects in males.[177] Bicalutamide neither suppresses nor inhibits androgen production in the body (i.e., it does not act as an antigonadotropin or androgen steroidogenesis inhibitor or lower androgen levels) and hence exclusively mediates its antiandrogenic effects by antagonizing the AR.[3][174][173] In addition to the classical nuclear AR, bicalutamide has been assessed at the membrane androgen receptors (mARs) and found to act as a potent antagonist of ZIP9 (IC50 = 66.3 nM), whereas it does not appear to interact with GPRC6A.[178][179]
The affinity of bicalutamide for the AR is relatively low as it is approximately 30 to 100 times lower than that of DHT, which is 2.5- to 10-fold as potent as an AR agonist as testosterone in bioassays and is the main endogenous ligand of the receptor in the prostate gland.[180][172][2][181] However, typical clinical dosages of bicalutamide result in circulating levels of the drug that are thousands of times higher than those of testosterone and DHT, allowing it to powerfully prevent them from binding to and activating the receptor.[182][183][34][184][27][89][185][13][186] This is especially true in the case of surgical or medical castration, in which testosterone levels in the circulation are approximately 95% reduced and DHT levels in the prostate gland are about 50 to 60% reduced.[172][187] In women, levels of testosterone are substantially lower (20- to 40-fold) than in men,[188] so much smaller doses of bicalutamide (e.g., 25 mg/day in the hirsutism studies) are necessary.[17][52][189][33]
Blockade of the AR by bicalutamide in the pituitary gland and hypothalamus results in prevention of the negative feedback of androgens on the hypothalamic–pituitary–gonadal axis (HPG axis) in males and consequent disinhibition of pituitary luteinizing hormone (LH) secretion.[109] This, in turn, results in an increase in circulating LH levels and activation of the gonadal production of testosterone and by extension production of estradiol.[190] Levels of testosterone have been found to increase 1.5- to 2-fold (59–97% increase) and levels of estradiol about 1.5- to 2.5-fold (65–146% increase) in men treated with 150 mg/day bicalutamide monotherapy.[31][32][33] In addition to testosterone and estradiol, there are smaller increases in concentrations of DHT, sex hormone-binding globulin, and prolactin.[33] Estradiol levels with bicalutamide monotherapy are similar to those in the low-normal premenopausal female range while testosterone levels generally remain in the high end of the normal male range.[32][191][174] Testosterone concentrations do not typically exceed the normal male range due to negative feedback on the HPG axis by the increased concentrations of estradiol.[109] Bicalutamide influences the HPG axis and increases hormone levels only in men and not also in women.[192][193][194] This is due to the much lower levels of androgens in women and their lack of basal suppression of the HPG axis in this sex.[192][193][194] As evidenced by its effectiveness in the treatment of prostate cancer and other androgen-dependent conditions, the antiandrogenic actions of bicalutamide considerably exceed any impact of the increased levels of testosterone it results in.[84] However, the elevated levels of estradiol remain unopposed by bicalutamide and are responsible for the gynecomastia and feminizing side effects it causes in men.[195] Although bicalutamide monotherapy increases gonadotropin and sex hormone levels in men, this will not occur if bicalutamide is combined with an antigonadotropin such as a GnRH analogue, estrogen, or progestogen, as these medications maintain negative feedback on the HPG axis.[50][196][197]
NSAA monotherapy, including with bicalutamide, shows a number of tolerability differences from methods of androgen deprivation therapy that incorporate surgical or medical castration. For example, the rates of hot flashes, depression, fatigue, and sexual dysfunction are all much higher with GnRH analogues than with NSAA monotherapy. It is thought that this is because GnRH analogues suppress estrogen production in addition to androgen production, resulting in estrogen deficiency.[198][199][200] In contrast, NSAA monotherapy does not decrease estrogen levels and in fact increases them, resulting in an excess of estrogens that compensates for androgen deficiency and allows for a preservation of mood, energy, and sexual function.[198][199][200] Neurosteroids that are produced from testosterone like 3α-androstanediol and 3β-androstanediol, which are ERβ agonists and the former a potent GABAA receptor positive allosteric modulator, may also be involved.[201][202][203][204][205][206][207] In the specific case of sexual dysfunction, an additional possibility for the difference is that without concomitant suppression of androgen production, blockade of the AR by the bicalutamide in the brain is incomplete and insufficient to markedly influence sexual function.[citation needed]
Under normal circumstances, bicalutamide has no capacity to activate the AR.[208][209] However, in prostate cancer, mutations and overexpression of the AR can accumulate in prostate gland cells which can convert bicalutamide from an antagonist of the AR into an agonist.[208][210] This can result in paradoxical stimulation of prostate cancer growth with bicalutamide and is responsible for the phenomenon of the antiandrogen withdrawal syndrome, where antiandrogen discontinuation paradoxically slows the rate of prostate cancer growth.[208][210]
In transgender women, breast development is a desired effect of antiandrogen or estrogen treatment.[63][211] Breast development and gynecomastia induced by bicalutamide is thought to be mediated by increased activation of the ER secondary to blockade of the AR (resulting in disinhibition of the ER in breast tissue) and increased levels of estradiol.[20][212][213] In addition to fat deposition, connective tissue growth, and ductal development, bicalutamide has been found to produce moderate lobuloalveolar development of the breasts.[214][215][216] However, full lobuloalveolar maturation necessary for lactation and breastfeeding will not occur without progestogen treatment.[214][215][216]
Bicalutamide monotherapy seems to have minimal effect on testicular spermatogenesis, testicular ultrastructure, and certain aspects of male fertility.[217][90][218] This seems to be because testosterone levels in the testes (where ~95% of testosterone in males is produced) are extremely high (up to 200-fold higher than circulating levels) and only a small fraction (less than 10%) of the normal levels of testosterone in the testes are actually necessary to maintain spermatogenesis.[219][220][221] As a result, bicalutamide appears to not be able to compete with testosterone in this sole part of the body to an extent sufficient to considerably interfere with androgen signaling and function.[219][220][221] However, while bicalutamide does not seem to be able to adversely influence testicular spermatogenesis, it may interfere with AR-dependent sperm maturation and transport outside of the testes in the epididymides and vas deferens where androgen levels are far lower, and hence may still be able to impair male fertility.[222] In addition, the combination of bicalutamide with other medications, such as estrogens, progestogens, and GnRH analogues, can compromise spermatogenesis due to their own adverse effects on male fertility.[223][224][225][226][227][228] These medications are able to strongly suppress gonadal androgen production, which can severely impair or abolish testicular spermatogenesis, and estrogens also appear to have direct and potentially long-lasting cytotoxic effects in the testes at sufficiently high concentrations.[223][224][225][226][227][228]
Other activities
[edit]Bicalutamide has been found to act as an inhibitor or inducer of certain cytochrome P450 enzymes including CYP3A4, CYP2C9, CYP2C19, and CYP2D6 in preclinical research, but no evidence of this has been found in humans treated with up to 150 mg/day.[2] It has also been identified in vitro as a strong inhibitor of CYP27A1 (cholesterol 27-hydroxylase) and as an inhibitor of CYP46A1 (cholesterol 24-hydroxylase), but this has yet to be assessed or confirmed in vivo or in humans and the clinical significance remains unknown.[229][230] Bicalutamide has been found to be a P-glycoprotein (ABCB1) inhibitor.[231][232][233] Like other first-generation NSAAs and enzalutamide, it has been found to act as a weak non-competitive inhibitor of GABAA receptor-mediated currents in vitro (IC50 = 5.2 μM).[234][235] However, unlike enzalutamide, bicalutamide has not been found to be associated with seizures or other related adverse central effects, so the clinical relevance of this finding is uncertain.[234][235]
Pharmacokinetics
[edit]Though its absolute bioavailability in humans is unknown, bicalutamide is known to be extensively and well-absorbed.[2][3] Its absorption is not affected by food.[3][167] The absorption of bicalutamide is linear at doses up to 150 mg/day and is saturable at doses above this, with no further increases in steady-state levels of bicalutamide occurring at doses above 300 mg/day.[2][13][236][164] Whereas absorption of (R)-bicalutamide is slow, with levels peaking at 31 to 39 hours after a dose, (S)-bicalutamide is much more rapidly absorbed.[13][27][2] Steady-state concentrations of the drug are reached after 4 to 12 weeks of treatment independently of dosage, with a 10- to 20-fold progressive accumulation in levels of (R)-bicalutamide.[13][237][238][185] The long time to steady-state levels is the result of bicalutamide's very long elimination half-life.[185] There is wide interindividual variability in (R)-bicalutamide levels (up to 16-fold) with bicalutamide regardless of dosage.[2]
The tissue distribution of bicalutamide is not well-characterized.[239] The amount of bicalutamide in semen that could potentially be transferred to a female partner during sexual intercourse is low and is not thought to be important.[89] Based on animal studies with rats and dogs it was thought that bicalutamide could not cross the blood–brain barrier and hence could not enter the brain.[240][34][241][242] As such, it was initially thought to be a peripherally selective antiandrogen.[240][34] However, subsequent clinical studies found that this was not also the case for humans, indicating species differences; bicalutamide crosses into the human brain and, in accordance, produces effects and side effects consistent with central antiandrogenic action.[2][109][243][244][245] In any case, there is indication that bicalutamide might have at least some peripheral selectivity in humans.[246] Bicalutamide is highly plasma protein bound (96.1% for racemic bicalutamide, 99.6% for (R)-bicalutamide) and is bound mainly to albumin, with negligible binding to SHBG and corticosteroid-binding globulin.[4][2][239][176]
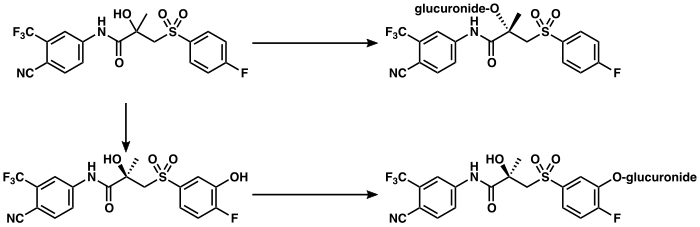
Bicalutamide is metabolized in the liver.[4][167] (R)-Bicalutamide is metabolized slowly and almost exclusively via hydroxylation by CYP3A4 into (R)-hydroxybicalutamide.[167][2][4][247] This metabolite is then glucuronidated by UGT1A9.[167][2][9][6] In contrast to (R)-bicalutamide, (S)-bicalutamide is metabolized rapidly and mainly by glucuronidation (without hydroxylation).[167] None of the metabolites of bicalutamide are known to be active and levels of the metabolites are low in plasma, where unchanged biclautamide predominates.[4][5][2] Due to the stereoselective metabolism of bicalutamide, (R)-bicalutamide has a far longer terminal half-life than (S)-bicalutamide and its levels are about 10- to 20-fold higher in comparison following a single dose and 100-fold higher at steady-state.[13][247][248] (R)-Bicalutamide has a relatively long elimination half-life of 5.8 days with a single dose and 7 to 10 days following repeated administration.[8]
Bicalutamide is eliminated in similar proportions in feces (43%) and urine (34%), while its metabolites are eliminated roughly equally in urine and bile.[4][167][249][250] The drug is excreted to a substantial extent in unmetabolized form, and both bicalutamide and its metabolites are eliminated mainly as glucuronide conjugates.[173] The glucuronide conjugates of bicalutamide and its metabolites are eliminated from the circulation rapidly, unlike unconjugated bicalutamide.[2][167][251]
The pharmacokinetics of bicalutamide are not affected by consumption of food, a person's age or body weight, renal impairment, or mild-to-moderate hepatic impairment.[2][185] However, steady-state levels of bicalutamide are higher in Japanese individuals than in white people.[2]
Chemistry
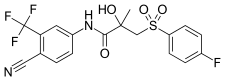
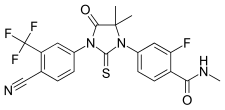
[edit]Bicalutamide is a racemic mixture consisting of equal proportions of enantiomers (R)-bicalutamide (dextrorotatory) and (S)-bicalutamide (levorotatory).[27] Its systematic name (IUPAC) is (RS)-N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-methylpropanamide.[252][253] The compound has a chemical formula of C18H14F4N2O4S, a molecular weight of 430.373 g/mol, and is a fine white to off-white powder.[27][89]
The acid dissociation constant (pKa') of bicalutamide is approximately 12.[89] It is a highly lipophilic compound (log P = 2.92).[2][254] At 37 °C (98.6 °F), or normal human body temperature, bicalutamide is practically insoluble in water (4.6 mg/L), acid (4.6 mg/L at pH 1), and alkali (3.7 mg/L at pH 8).[27][89] In organic solvents, it is slightly soluble in chloroform and absolute ethanol, sparingly soluble in methanol, and freely soluble in acetone and tetrahydrofuran.[27][89]
Bicalutamide is a synthetic and nonsteroidal compound which was derived from flutamide.[255] It is a bicyclic compound (has two rings) and can be classified as and has variously been referred to as an anilide (N-phenylamide) or aniline, a diarylpropionamide, and a toluidide.[255][247]
Analogues
[edit]First-generation NSAAs including bicalutamide, flutamide, and nilutamide are all synthetic, nonsteroidal anilide derivatives and structural analogues of each other.[255] Bicalutamide is a diarylpropionamide while flutamide is a monoarylpropionamide and nilutamide is a hydantoin.[255] Bicalutamide and flutamide, though not nilutamide, can also be classified as toluidides.[247] All three of the compounds share a common 3-trifluoromethylaniline moiety.[256] Bicalutamide is a modification of flutamide in which a 4-fluorophenylsulfonyl moiety has been added and the nitro group on the original phenyl ring has been replaced with a cyano group.[257] Topilutamide, also known as fluridil, is another NSAA that is closely related structurally to the first-generation NSAAs, but, in contrast to them, is not used in the treatment of prostate cancer and is instead used exclusively as a topical antiandrogen in the treatment of pattern hair loss.[258][259][260]
-
Bicalutamide
The second-generation NSAAs enzalutamide and apalutamide were derived from and are analogues of the first-generation NSAAs,[167][261] while another second-generation NSAA, darolutamide, is said to be structurally distinct and chemically unrelated to the other NSAAs.[262] Enzalutamide is a modification of bicalutamide in which the inter-ring linking chain has been altered and cyclized into a 5,5-dimethyl-4-oxo-2-thioxo imidazolidine moiety. In apalutamide, the 5,5-dimethyl groups of the imidazolidine ring of enzalutamide are cyclized to form an accessory cyclobutane ring and one of its phenyl rings is replaced with a pyridine ring.
The first nonsteroidal androgens, the arylpropionamides, were discovered via structural modification of bicalutamide.[263] Unlike bicalutamide (which is purely antiandrogenic), these compounds show tissue-selective androgenic effects and were classified as selective androgen receptor modulators (SARMs).[263] Lead SARMs of this series included acetothiolutamide, enobosarm (ostarine; S-22), and andarine (acetamidoxolutamide or androxolutamide; S-4).[255][263][264] They are very close to bicalutamide structurally, with the key differences being that the linker sulfone of bicalutamide has been replaced with an ether or thioether group to confer agonism of the AR and the 4-fluoro atom of the pertinent phenyl ring has been substituted with an acetamido or cyano group to eliminate reactivity at the position.[265]
A few radiolabeled derivatives of bicalutamide have been developed for potential use as radiotracers in medical imaging.[266][267] They include [18F]bicalutamide, 4-[76Br]bromobicalutamide, and [76Br]bromo-thiobicalutamide.[266][267] The latter two were found to have substantially increased affinity for the AR relative to that of bicautamide.[266] However, none of these agents have been evaluated in humans.[266][267]
5N-Bicalutamide, or 5-azabicalutamide, is a minor structural modification of bicalutamide which acts as a reversible covalent antagonist of the AR and has approximately 150-fold higher affinity for the AR and about 20-fold greater functional inhibition of the AR relative to bicalutamide.[268][269] It is among the most potent AR antagonists to have been developed and is being researched for potential use in the treatment of antiandrogen-resistant prostate cancer.[268]
Synthesis
[edit]A number of chemical syntheses of bicalutamide have been published in the literature.[252][270][271][272][273] The procedure of the first published synthesis of bicalutamide can be seen below.[270]
Bicalutamide synthesis[270]
|
History
[edit]Bicalutamide as well as all of the other currently marketed NSAAs were derived from structural modification of flutamide, which itself was originally synthesized as a bacteriostatic agent in 1967 at Schering Plough Corporation and was subsequently and serendipitously found to possess antiandrogenic activity.[274][275][276] Bicalutamide was discovered by Tucker and colleagues at Imperial Chemical Industries (ICI) in the 1980s and was selected for development from a group of over 2,000 synthesized compounds.[277][176][278][252] It was first patented in 1982[279] and was first reported in the scientific literature in June 1987.[280]
Bicalutamide was first studied in a phase I clinical trial in 1987[107] and the results of the first phase II clinical trial in prostate cancer were published in 1990.[281] The pharmaceutical division of ICI was split out into an independent company called Zeneca in 1993, and in April and May 1995, Zeneca (now AstraZeneca, after merging with Astra AB in 1999) began pre-approval marketing of bicalutamide for the treatment of prostate cancer in the U.S..[282] It was first launched in the U.K. in May 1995,[283] and was subsequently approved by the U.S. FDA on 4 October 1995, for the treatment of prostate cancer at a dosage of 50 mg/day in combination with a GnRH analogue.[284][285]
Following its introduction for use in combination with a GnRH analogue, bicalutamide was developed as a monotherapy at a dosage of 150 mg/day for the treatment of prostate cancer, and was approved for this indication in Europe, Canada, and a number of other countries in the late 1990s and early 2000s.[13][172][286][287] This application of bicalutamide was also under review by the FDA in the U.S. in 2002,[288] but ultimately was not approved in this country.[84] In Japan, bicalutamide is licensed at a dosage of 80 mg/day alone or in combination with a GnRH analogue for prostate cancer.[48] The unique 80 mg dosage of bicalutamide used in Japan was selected for development in this country on the basis of observed pharmacokinetic differences with bicalutamide in Japanese men.[49]
Subsequent to negative findings of bicalutamide monotherapy for LPC in the EPC clinical programme, approval of bicalutamide for use specifically in the treatment of LPC was withdrawn in a number of countries[14] including the U.K. (in October or November 2003)[15] and several other European countries and Canada (in August 2003).[13][289][290] In addition, the U.S. and Canada explicitly recommended against the use of 150 mg/day bicalutamide for this indication.[16] The drug is effective for, remains approved for, and continues to be used in the treatment of LAPC and mPC, on the other hand.[13]
The patent protection of bicalutamide expired in the U.S. in March 2009 and the drug has subsequently been available as a generic,[291] at greatly reduced cost.[292]
Bicalutamide was the fourth antiandrogen (and the third NSAA) to be introduced for the treatment of prostate cancer, following the SAA CPA in 1973[293] and the NSAAs flutamide in 1983 (1989 in the U.S.)[252][294] and nilutamide in 1989 (1996 in the U.S.).[256][295][296] It has been followed by abiraterone acetate in 2011, enzalutamide in 2012, apalutamide in 2018, and darolutamide in 2019, and may also be followed by in-development drugs such as proxalutamide and seviteronel.[297]
Society and culture
[edit]Generic names
[edit]Bicalutamide is the generic name of the drug in English and French and its INN, USAN, USP,[298] BAN, DCF, AAN,[89] and JAN.[38][299][80][300] It is also referred to as bicalutamidum in Latin, bicalutamida in Spanish and Portuguese, bicalutamid in German, and bikalutamid in Russian and other Slavic languages.[38][80] The "bica-" prefix corresponds to the fact that bicalutamide is a bicyclic compound, while the "-lutamide" suffix is the standard suffix for NSAAs.[301][302] Bicalutamide is also known by its former developmental code name ICI-176,334.[299][80][38]
Brand names
[edit]Bicalutamide is marketed by AstraZeneca in oral tablet form under the brand names Casodex, Cosudex, Calutide, Calumid, and Kalumid in many countries.[38][80][303][304] It is also marketed under the brand names Bicadex, Bical, Bicalox, Bicamide, Bicatlon, Bicusan, Binabic, Bypro, Calutol, and Ormandyl among others in various countries.[38] The drug is sold under a large number of generic trade names such as Apo-Bicalutamide, Bicalutamide Accord, Bicalutamide Actavis, Bicalutamide Bluefish, Bicalutamide Kabi, Bicalutamide Sandoz, and Bicalutamide Teva as well.[38] A combination formulation of bicalutamide and goserelin is marketed by AstraZeneca in Australia and New Zealand under the brand name ZolaCos-CP.[81][86][87][88]
Cost and generics
[edit]Bicalutamide is off-patent and available as a generic.[291] Unlike bicalutamide, the newer NSAA enzalutamide is still on-patent, and for this reason, is considerably more expensive in comparison.[305]
The patent protection of all three of the first-generation NSAAs has expired and flutamide and bicalutamide are both available as low-cost generics.[306][307] Nilutamide, on the other hand, has always been a poor third competitor to flutamide and bicalutamide and, in relation to this fact, has not been developed as a generic and is only available as brand name Nilandron, at least in the U.S.[306][307]
Bicalutamide is considerably less costly than GnRH analogues, which, in spite of some having been off-patent many years, have been reported (in 2013) to typically cost US$10,000–$15,000 per year (or about US$1,000 per month) of treatment.[308][309]
Sales and usage
[edit]Sales of bicalutamide (as Casodex) worldwide peaked at US$1.3 billion in 2007,[310] and it has been described as a "billion-dollar-a-year" drug prior to losing its patent protection starting in 2007.[43][311][258] In 2014, despite the introduction of abiraterone acetate in 2011 and enzalutamide in 2012, bicalutamide was still the most commonly prescribed drug in the treatment of metastatic castration-resistant prostate cancer (mCRPC).[43] Moreover, in spite of being off-patent, bicalutamide was said to still generate a few hundred million dollars in sales per year for AstraZeneca.[43] Total worldwide sales of brand name Casodex were approximately US$13.4 billion as of the end of 2018.[312][313][40][314][315][310][316][317][318][319][320][excessive citations]
| Year | Sales | Year | Sales | Year | Sales | Year | Sales | Year | Sales | Year | Sales | Year | Sales | Year | Sales |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1995 | ~$15m | 1998 | $245m | 2001 | $569m | 2004 | $1012m | 2007* | $1335m | 2010 | $579m | 2013 | $376m | 2016 | $247m |
| 1996 | $109m | 1999 | $340m | 2002 | $644m | 2005 | $1123m | 2008 | $1258m | 2011 | $550m | 2014 | $320m | 2017 | $215m |
| 1997 | $200m | 2000 | $433m | 2003 | $854m | 2006 | $1206m | 2009 | $844m | 2012 | $454m | 2015 | $267m | 2018 | $201m |
| Notes: First generic availability (*) was in 2007.[311] Total sales as of end 2018 were $13.4 billion. Sources:[312][313][40][314][315][310][316][317][318][319][320] | |||||||||||||||
Between January 2007 and December 2009 (a period of three years), 1,232,143 prescriptions of bicalutamide were dispensed in the U.S., or about 400,000 prescriptions per year.[44] During that time, bicalutamide accounted for about 87.2% of the NSAA market, while flutamide accounted for 10.5% of it and nilutamide for 2.3% of it.[44] Approximately 96% of bicalutamide prescriptions were written for diagnosis codes that clearly indicated neoplasm.[44] About 1,200, or 0.1% of bicalutamide prescriptions were dispensed to pediatric patients (age 0–16).[44]
Regulation
[edit]Bicalutamide is a prescription drug.[83] It is not specifically a controlled substance in any country and therefore is not an illegal drug.[10] However, the manufacture, sale, distribution, and possession of prescription drugs are all still subject to legal regulation throughout the world.[321][322][323]
Research
[edit]Bicalutamide has been studied in combination with the 5α-reductase inhibitors finasteride and dutasteride in prostate cancer.[324][325][326][327][328][329][330] It has also been studied in combination with raloxifene, a selective estrogen receptor modulator (SERM), for the treatment of prostate cancer.[331][332] Bicalutamide has been tested for the treatment of AR-positive ER/PR-negative locally advanced and metastatic breast cancer in women in a phase II study for this indication.[333][334][335] Enzalutamide is also being investigated for this type of cancer.[336][337] Bicalutamide has also been studied in a phase II clinical trial for ovarian cancer in women.[338]
Bicalutamide has been studied in the treatment of benign prostatic hyperplasia (BPH) in a 24-week trial of 15 patients at a dosage of 50 mg/day.[339][340] Prostate volume decreased by 26% in patients taking bicalutamide and urinary irritative symptom scores significantly decreased.[339][340] Conversely, peak urine flow rates and urine pressure flow examinations were not significantly different between bicalutamide and placebo.[339][340] The decrease in prostate volume achieved with bicalutamide was comparable to that observed with the 5α-reductase inhibitor finasteride, which is approved for the treatment of BPH.[341][342] Breast tenderness (93%), gynecomastia (54%), and sexual dysfunction (60%) were all reported as side effects of bicalutamide at the dosage used in the study, although no treatment discontinuations due to adverse effects occurred and sexual functioning was maintained in 75% of patients.[340][107]
A phase III clinical trial of bicalutamide in combination with an ethinylestradiol-containing combined oral contraceptive for the treatment of severe hirsutism in women with PCOS was completed in Italy in 2017 under supervision of the Italian Agency for Drugs (AIFA).[57]
Antiandrogens have been suggested for treating COVID-19 in men and as of May 2020 high-dose bicalutamide is in a phase II clinical trial for this purpose.[343][344]
Veterinary use
[edit]Bicalutamide may be used to treat hyperandrogenism and associated benign prostatic hyperplasia secondary to hyperadrenocorticism (caused by excessive adrenal androgens) in male ferrets.[345][346][347] However, it has not been formally assessed in controlled studies for this purpose.[347][348]
See also
[edit]References
[edit]- ^ a b Finkel R, Clark MA, Cubeddu LX (2009). Pharmacology. Lippincott Williams & Wilkins. pp. 481–. ISBN 978-0-7817-7155-9.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai Cockshott ID (2004). "Bicalutamide: clinical pharmacokinetics and metabolism". Clinical Pharmacokinetics. 43 (13): 855–878. doi:10.2165/00003088-200443130-00003. PMID 15509184. S2CID 29912565.
- ^ a b c d e f g h i j Dart RC (2004). Medical Toxicology. Lippincott Williams & Wilkins. pp. 497, 521. ISBN 978-0-7817-2845-4. Archived from the original on 11 May 2016.
- ^ a b c d e f g h i j k l m n Lemke TL, Williams DA (2008). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 121, 1288, 1290. ISBN 978-0-7817-6879-5. Archived from the original on 8 September 2017.
- ^ a b Dole EJ, Holdsworth MT (1997). "Nilutamide: an antiandrogen for the treatment of prostate cancer". The Annals of Pharmacotherapy. 31 (1): 65–75. doi:10.1177/106002809703100112. PMID 8997470. S2CID 20347526.
page 67: Currently, information is not available regarding the activity of the major urinary metabolites of bicalutamide, bicalutamide glucuronide, and hydroxybicalutamide glucuronide.
- ^ a b Schellhammer PF (September 2002). "An evaluation of bicalutamide in the treatment of prostate cancer". Expert Opinion on Pharmacotherapy. 3 (9): 1313–28. doi:10.1517/14656566.3.9.1313. PMID 12186624. S2CID 32216411.
The clearance of bicalutamide occurs pre- dominantly by hepatic metabolism and glucuronidation, with excretion of the resulting inactive metabolites in the urine and faces.
- ^ a b c Skidmore-Roth L (17 April 2013). Mosby's 2014 Nursing Drug Reference – Elsevieron VitalSource. Elsevier Health Sciences. pp. 193–194. ISBN 978-0-323-22267-9. Archived from the original on 14 January 2023. Retrieved 27 September 2016.
- ^ a b Jordan VC, Furr BJ (5 February 2010). Hormone Therapy in Breast and Prostate Cancer. Springer Science & Business Media. pp. 350–. ISBN 978-1-59259-152-7. Archived from the original on 29 May 2016.
- ^ a b c Grosse L, Campeau AS, Caron S, Morin FA, Meunier K, Trottier J, Caron P, Verreault M, Barbier O (August 2013). "Enantiomer selective glucuronidation of the non-steroidal pure anti-androgen bicalutamide by human liver and kidney: role of the human UDP-glucuronosyltransferase (UGT)1A9 enzyme". Basic & Clinical Pharmacology & Toxicology. 113 (2): 92–102. doi:10.1111/bcpt.12071. PMC 3815647. PMID 23527766.
- ^ a b c d e f g h i j "Bicalutamide". The American Society of Health-System Pharmacists. Archived from the original on 29 December 2016. Retrieved 8 December 2016.
- ^ Wass JA, Stewart PM (28 July 2011). Oxford Textbook of Endocrinology and Diabetes. OUP Oxford. pp. 1625–. ISBN 978-0-19-923529-2. Archived from the original on 11 May 2016.
- ^ Shergill I, Arya M, Grange PR, Mundy AR (2010). Medical Therapy in Urology. Springer Science & Business Media. p. 40. ISBN 9781848827042. Archived from the original on 28 October 2014.
- ^ a b c d e f g h i j k l m n o p q r s t Wellington K, Keam SJ (2006). "Bicalutamide 150mg: a review of its use in the treatment of locally advanced prostate cancer" (PDF). Drugs. 66 (6): 837–50. doi:10.2165/00003495-200666060-00007. PMID 16706554. S2CID 46966712. Archived from the original (PDF) on 28 August 2016. Retrieved 13 August 2016.
- ^ a b Shahani R, Fleshner NE, Zlotta AR (2007). "Pharmacotherapy for prostate cancer: the role of hormonal treatment". Discovery Medicine. 7 (39): 118–24. PMID 18093474. Archived from the original on 9 August 2020. Retrieved 13 August 2016.
- ^ a b Bowsher W, Carter A (15 April 2008). Challenges in Prostate Cancer. John Wiley & Sons. pp. 146–. ISBN 978-1-4051-7177-9.
- ^ a b Nargund VH, Raghavan D, Sandler HM (17 January 2015). Urological Oncology. Springer. pp. 823–. ISBN 978-0-85729-482-1.
On the other hand, the 150 mg dose of bicalutamide has been associated with some safety concerns, such as a higher death rate when added to active surveillance in the early prostate cancer trialists group study [29], which has led the United States and Canada to recommend against prescribing the 150 mg dose [30].
- ^ a b c Williams H, Bigby M, Diepgen T, Herxheimer A, Naldi L, Rzany B (22 January 2009). Evidence-Based Dermatology. John Wiley & Sons. pp. 529–. ISBN 978-1-4443-0017-8. Archived from the original on 2 May 2016.
- ^ a b Carvalho RM, Santos LD, Ramos PM, Machado CJ, Acioly P, Frattini SC, Barcaui CB, Donda AL, Melo DF (January 2022). "Bicalutamide and the new perspectives for female pattern hair loss treatment: What dermatologists should know". J Cosmet Dermatol. 21 (10): 4171–4175. doi:10.1111/jocd.14773. PMID 35032336. S2CID 253239337.
- ^ a b Randolph JF (December 2018). "Gender-Affirming Hormone Therapy for Transgender Females". Clin Obstet Gynecol. 61 (4): 705–721. doi:10.1097/GRF.0000000000000396. PMID 30256230. S2CID 52821192.
- ^ a b c Jameson JL, De Groot LJ (25 February 2015). Edndocrinology: Adult and Pediatric. Elsevier Health Sciences. pp. 2425–2426, 2139. ISBN 978-0-323-32195-2.
- ^ a b Yuan J, Desouza R, Westney OL, Wang R (2008). "Insights of priapism mechanism and rationale treatment for recurrent priapism". Asian Journal of Andrology. 10 (1): 88–101. doi:10.1111/j.1745-7262.2008.00314.x. PMID 18087648.
- ^ a b Elliott S, Latini DM, Walker LM, Wassersug R, Robinson JW (2010). "Androgen deprivation therapy for prostate cancer: recommendations to improve patient and partner quality of life". The Journal of Sexual Medicine. 7 (9): 2996–3010. doi:10.1111/j.1743-6109.2010.01902.x. PMID 20626600.
- ^ a b Hammerer P, Manka L (2019). "Androgen Deprivation Therapy for Advanced Prostate Cancer". Urologic Oncology. Springer International Publishing. pp. 255–276. doi:10.1007/978-3-319-42623-5_77. ISBN 978-3-319-42622-8.
Bicalutamide is the most widely used antiandrogen in the treatment of prostate cancer. [...] Common side effects [of bicalutamide] include breast enlargement, breast tenderness, hot flashes, and constipation as well as feminization and changes in mood and liver as well as lung toxicity; monitoring of liver enzymes is recommended during treatment (Schellhammer and Davis 2004).
- ^ Droz J, Audisio RA (2 October 2012). Management of Urological Cancers in Older People. Springer Science & Business Media. pp. 84–. ISBN 978-0-85729-986-4. Archived from the original on 11 May 2016.
- ^ a b Shapiro J (12 November 2012). Hair Disorders: Current Concepts in Pathophysiology, Diagnosis and Management, An Issue of Dermatologic Clinics. Elsevier Health Sciences. pp. 187–. ISBN 978-1-4557-7169-1.
- ^ a b Jia AY, Spratt DE (June 2022). "Bicalutamide Monotherapy With Radiation Therapy for Localized Prostate Cancer: A Non-Evidence-Based Alternative". Int J Radiat Oncol Biol Phys. 113 (2): 316–319. doi:10.1016/j.ijrobp.2022.01.037. PMID 35569476. S2CID 248765294.
Four other randomized trials using BICmono have also raised concerns about either lack of efficacy or even harm from this treatment approach compared with placebo or no hormone therapy. SPCG-6 randomized 1218 patients to either 150 mg of BICmono daily or placebo. In the subset of patients with LPCa managed with observation, survival was significantly worse with BIC than placebo (hazard ratio [HR], 1.47; 95% confidence interval, 1.06-2.03).10 Two other randomized trials were part of the early prostate cancer program,11 which conducted 3 randomized trials that were pooled together to determine the benefit of BICmono (SPCG-6 was one of the 3 trials). Overall, in the combined 8113 patient pooled cohort, after a median follow-up of 7 years, there was no improvement even in progression-free survival from the use of adjuvant BIC in LPCa, and there was a trend for worse overall survival (HR, 1.16; 95% confidence interval, 0.99-1.37; P = .07). [...] Although not in LPCa, NRG/RTOG 9601 demonstrated findings consistent with the prior trials.12 This trial randomized men to postprostatectomy salvage radiation therapy plus placebo versus 150 mg of BICmono daily for 2 years. After a median follow-up of 13 years, the trial showed that there were significantly more grade 3 to 5 cardiac events in the BICmono arm. In patients with less aggressive disease with lower PSAs (prostate-specific antigens; more analogous to LPCa), other-cause mortality was significantly higher in the BICmono arm. In patients with high PSAs >1.5 ng/mL (which with modern molecular positron emission tomography imaging would be expected to have high rates of regional and distant metastatic disease), a survival benefit from the addition of BIC was observed. This is consistent with results from the early prostate cancer studies that showed that only patients with more advanced disease derived benefit from BICmono.10 Thus, all the randomized evidence from 5 trials (Table 1) demonstrates that, in LPCa, BICmono had no clinically significant oncologic activity over placebo/no treatment, and consistent trends with long-term use resulted in worse survival.
- ^ a b c d e f g h i j k l m n o p q r "Casodex- bicalutamide tablet". DailyMed. 1 September 2019. Archived from the original on 27 July 2020. Retrieved 7 May 2020.
- ^ a b Lee K, Oda Y, Sakaguchi M, Yamamoto A, Nishigori C (May 2016). "Drug-induced photosensitivity to bicalutamide – case report and review of the literature". Photodermatology, Photoimmunology & Photomedicine. 32 (3): 161–4. doi:10.1111/phpp.12230. PMID 26663090. S2CID 2761388.
- ^ Lee K, et al. (2016). "Drug-induced photosensitivity to bicalutamide – case report and review of the literature". Reactions Weekly. 1612 (1): 161–4. doi:10.1007/s40278-016-19790-1. PMID 26663090. S2CID 261402820.
- ^ a b Singh SM, Gauthier S, Labrie F (February 2000). "Androgen receptor antagonists (antiandrogens): structure-activity relationships". Current Medicinal Chemistry. 7 (2): 211–47. doi:10.2174/0929867003375371. PMID 10637363.
- ^ a b Strauss III JF, Barbieri RL (28 August 2013). Yen & Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Elsevier Health Sciences. pp. 688–. ISBN 978-1-4557-5972-9. Archived from the original on 14 January 2023. Retrieved 27 September 2016.
Bone density improves in men receiving bicalutamide, most likely secondary to the 146% increase in estradiol and the fact that estradiol is the major mediator of bone density in men.
- ^ a b c Marcus R, Feldman D, Nelson D, Rosen CJ (8 November 2007). Osteoporosis. Academic Press. pp. 1354–. ISBN 978-0-08-055347-4. Archived from the original on 11 June 2016.
- ^ a b c d e Mahler C, Verhelst J, Denis L (May 1998). "Clinical pharmacokinetics of the antiandrogens and their efficacy in prostate cancer". Clinical Pharmacokinetics. 34 (5): 405–17. doi:10.2165/00003088-199834050-00005. PMID 9592622. S2CID 25200595.
- ^ a b c d e Furr BJ, Tucker H (January 1996). "The preclinical development of bicalutamide: pharmacodynamics and mechanism of action". Urology. 47 (1A Suppl): 13–25, discussion 29–32. doi:10.1016/S0090-4295(96)80003-3. PMID 8560673.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 515. ISBN 9783527607495. Archived from the original on 12 January 2023. Retrieved 24 August 2017.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Hamilton R (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 381. ISBN 9781284057560.
- ^ a b c d e f g h "Bicalutamide – International Drug Names". Drugs.com. Archived from the original on 18 September 2016. Retrieved 13 August 2016.
- ^ a b Akaza H (1999). "[A new anti-androgen, bicalutamide (Casodex), for the treatment of prostate cancer—basic clinical aspects]". Gan to Kagaku Ryoho. Cancer & Chemotherapy (in Japanese). 26 (8): 1201–7. PMID 10431591.
- ^ a b c d "1999 Annual Report and Form 20-F" (PDF). AstraZeneca. Archived (PDF) from the original on 9 September 2017. Retrieved 1 July 2017.
- ^ Mukherji D, Pezaro CJ, De-Bono JS (February 2012). "MDV3100 for the treatment of prostate cancer". Expert Opinion on Investigational Drugs. 21 (2): 227–33. doi:10.1517/13543784.2012.651125. PMID 22229405. S2CID 46339544.
- ^ Pchejetski D, Alshaker H, Stebbing J (2014). "Castrate-resistant prostate cancer: the future of antiandrogens" (PDF). Trends in Urology & Men's Health. 5 (1): 7–10. doi:10.1002/tre.371. S2CID 57988002. Archived (PDF) from the original on 19 July 2018. Retrieved 11 December 2019.
- ^ a b c d Campbell T (22 January 2014). "Slowing Sales for Johnson & Johnson's Zytiga May Be Good News for Medivation". The Motley Fool. Archived from the original on 26 August 2016. Retrieved 20 July 2016.
[...] the most commonly prescribed treatment for metastatic castration resistant prostate cancer: bicalutamide. That was sold as AstraZeneca's billion-dollar-a-year drug Casodex before losing patent protection in 2008. AstraZeneca still generates a few hundred million dollars in sales from Casodex, [...]
- ^ a b c d e f Chang S (10 March 2010), Bicalutamide BPCA Drug Use Review in the Pediatric Population (PDF), U.S. Department of Health and Human Service, archived (PDF) from the original on 24 October 2016, retrieved 20 July 2016
- ^ a b Bagatelle C, Bremner WJ (27 May 2003). Androgens in Health and Disease. Springer Science & Business Media. pp. 25–. ISBN 978-1-59259-388-0.
- ^ Klotz L, Schellhammer P (March 2005). "Combined androgen blockade: the case for bicalutamide". Clinical Prostate Cancer. 3 (4): 215–9. doi:10.3816/cgc.2005.n.002. PMID 15882477.
- ^ Schellhammer PF, Sharifi R, Block NL, Soloway MS, Venner PM, Patterson AL, Sarosdy MF, Vogelzang NJ, Schellenger JJ, Kolvenbag GJ (September 1997). "Clinical benefits of bicalutamide compared with flutamide in combined androgen blockade for patients with advanced prostatic carcinoma: final report of a double-blind, randomized, multicenter trial. Casodex Combination Study Group". Urology. 50 (3): 330–6. doi:10.1016/S0090-4295(97)00279-3. PMID 9301693.
- ^ a b c Suzuki H, Kamiya N, Imamoto T, Kawamura K, Yano M, Takano M, Utsumi T, Naya Y, Ichikawa T (October 2008). "Current topics and perspectives relating to hormone therapy for prostate cancer". International Journal of Clinical Oncology. 13 (5): 401–10. doi:10.1007/s10147-008-0830-y. PMID 18946750. S2CID 32859879.
- ^ a b Usami M, Akaza H, Arai Y, Hirano Y, Kagawa S, Kanetake H, Naito S, Sumiyoshi Y, Takimoto Y, Terai A, Yoshida H, Ohashi Y (2007). "Bicalutamide 80 mg combined with a luteinizing hormone-releasing hormone agonist (LHRH-A) versus LHRH-A monotherapy in advanced prostate cancer: findings from a phase III randomized, double-blind, multicenter trial in Japanese patients". Prostate Cancer Prostatic Dis. 10 (2): 194–201. doi:10.1038/sj.pcan.4500934. PMID 17199134.
In most countries, bicalutamide is given at a dose of 50 mg when used in combination with an LHRH-A. However, based on pharmacokinetic and pharmacodynamic data, the approved dose of bicalutamide in Japanese men is 80 mg per day.
- ^ a b Melmed S (1 January 2016). Williams Textbook of Endocrinology. Elsevier Health Sciences. pp. 752–. ISBN 978-0-323-29738-7.
GnRH analogues, both agonists and antagonists, severely suppress endogenous gonadotropin and testosterone production [...] Administration of GnRH agonists (e.g., leuprolide, goserelin) produces an initial stimulation of gonadotropin and testosterone secretion (known as a "flare"), which is followed in 1 to 2 weeks by GnRH receptor downregulation and marked suppression of gonadotropins and testosterone to castration levels. [...] To prevent the potential complications associated with the testosterone flare, AR antagonists (e.g., bicalutamide) are usually coadministered with a GnRH agonist for men with metastatic prostate cancer.399
- ^ Sugiono M, Winkler MH, Okeke AA, Benney M, Gillatt DA (2005). "Bicalutamide vs cyproterone acetate in preventing flare with LHRH analogue therapy for prostate cancer—a pilot study". Prostate Cancer and Prostatic Diseases. 8 (1): 91–4. doi:10.1038/sj.pcan.4500784. PMID 15711607.
- ^ a b c Erem C (2013). "Update on idiopathic hirsutism: diagnosis and treatment". Acta Clinica Belgica. 68 (4): 268–74. doi:10.2143/ACB.3267. PMID 24455796. S2CID 39120534.
- ^ Ascenso A, Marques HC (January 2009). "Acne in the adult". Mini Reviews in Medicinal Chemistry. 9 (1): 1–10. doi:10.2174/138955709787001730. PMID 19149656.
- ^ Kaur S, Verma P, Sangwan A, Dayal S, Jain VK (2016). "Etiopathogenesis and Therapeutic Approach to Adult Onset Acne". Indian Journal of Dermatology. 61 (4): 403–7. doi:10.4103/0019-5154.185703. PMC 4966398. PMID 27512185.
- ^ Lotti F, Maggi M (2015). "Hormonal Treatment for Skin Androgen-Related Disorders". European Handbook of Dermatological Treatments. Springer. pp. 1451–1464. doi:10.1007/978-3-662-45139-7_142. ISBN 978-3-662-45138-0.
- ^ Müderris II, Öner G (2009). "Hirsutizm Tedavisinde Flutamid ve Bikalutamid Kullanımı" [Flutamide and Bicalutamide Treatment in Hirsutism]. Turkiye Klinikleri Journal of Endocrinology-Special Topics (in Turkish). 2 (2): 110–2. ISSN 1304-0529. Archived from the original on 27 July 2020. Retrieved 28 March 2019.
- ^ a b c Moretti C, Guccione L, Di Giacinto P, Simonelli I, Exacoustos C, Toscano V, Motta C, De Leo V, Petraglia F, Lenzi A (March 2018). "Combined Oral Contraception and Bicalutamide in Polycystic Ovary Syndrome and Severe Hirsutism: A Double-Blind Randomized Controlled Trial". J. Clin. Endocrinol. Metab. 103 (3): 824–838. doi:10.1210/jc.2017-01186. PMID 29211888.
- ^ Fishman SL, Paliou M, Poretsky L, Hembree WC (2019). "Endocrine Care of Transgender Adults". Transgender Medicine. Contemporary Endocrinology. Springer. pp. 143–163. doi:10.1007/978-3-030-05683-4_8. ISBN 978-3-030-05682-7. ISSN 2523-3785. S2CID 86772102.
- ^ Neyman A, Fuqua JS, Eugster EA (April 2019). "Bicalutamide as an Androgen Blocker With Secondary Effect of Promoting Feminization in Male-to-Female Transgender Adolescents". The Journal of Adolescent Health. 64 (4): 544–546. doi:10.1016/j.jadohealth.2018.10.296. PMC 6431559. PMID 30612811.
- ^ Gooren LJ (March 2011). "Clinical practice. Care of transsexual persons". The New England Journal of Medicine. 364 (13): 1251–1257. doi:10.1056/nejmcp1008161. PMID 21449788.
- ^ Deutsch M (17 June 2016). Guidelines for the Primary and Gender-Affirming Care of Transgender and Gender Nonbinary People (PDF) (2nd ed.). University of California, San Francisco: Center of Excellence for Transgender Health. p. 28. Archived (PDF) from the original on 30 May 2023. Retrieved 9 June 2023.
- ^ Vincent B (21 June 2018). Transgender Health: A Practitioner's Guide to Binary and Non-Binary Trans Patient Care. Jessica Kingsley Publishers. pp. 158–. ISBN 978-1-78450-475-5. Archived from the original on 14 January 2023. Retrieved 1 January 2019.
- ^ a b Wierckx K, Gooren L, T'Sjoen G (May 2014). "Clinical review: Breast development in trans women receiving cross-sex hormones". The Journal of Sexual Medicine. 11 (5): 1240–1247. doi:10.1111/jsm.12487. PMID 24618412.
- ^ Schoelwer M, Eugster EA (2015). "Treatment of Peripheral Precocious Puberty". Puberty from Bench to Clinic. Endocrine Development. Vol. 29. pp. 230–239. doi:10.1159/000438895. ISBN 978-3-318-02788-4. PMC 5345994. PMID 26680582.
- ^ Haddad NG, Eugster EA (June 2019). "Peripheral precocious puberty including congenital adrenal hyperplasia: causes, consequences, management and outcomes". Best Practice & Research. Clinical Endocrinology & Metabolism. 33 (3): 101273. doi:10.1016/j.beem.2019.04.007. hdl:1805/19111. PMID 31027974. S2CID 135410503.
- ^ Haddad NG, Eugster EA (2012). "Peripheral Precocious Puberty: Interventions to Improve Growth". Handbook of Growth and Growth Monitoring in Health and Disease. Springer. pp. 1199–1212. doi:10.1007/978-1-4419-1795-9_71. ISBN 978-1-4419-1794-2.
- ^ Zacharin M (2019). "Disorders of Puberty: Pharmacotherapeutic Strategies for Management". Pediatric Pharmacotherapy. Handbook of Experimental Pharmacology. Vol. 261. Springer. pp. 507–538. doi:10.1007/164_2019_208. ISBN 978-3-030-50493-9. ISSN 0171-2004. PMID 31144045. S2CID 169040406.
- ^ Kliegman RM, Stanton B, St Geme J, Schor NF (17 April 2015). Nelson Textbook of Pediatrics. Elsevier Health Sciences. pp. 2661–. ISBN 978-0-323-26352-8. Archived from the original on 12 January 2023. Retrieved 27 September 2016.
- ^ Reiter EO, Mauras N, McCormick K, Kulshreshtha B, Amrhein J, De Luca F, et al. (October 2010). "Bicalutamide plus anastrozole for the treatment of gonadotropin-independent precocious puberty in boys with testotoxicosis: a phase II, open-label pilot study (BATT)". Journal of Pediatric Endocrinology & Metabolism. 23 (10): 999–1009. doi:10.1515/jpem.2010.161. PMID 21158211. S2CID 110630.
- ^ Levey HR, Kutlu O, Bivalacqua TJ (January 2012). "Medical management of ischemic stuttering priapism: a contemporary review of the literature". Asian Journal of Andrology. 14 (1): 156–163. doi:10.1038/aja.2011.114. PMC 3753435. PMID 22057380.
- ^ Broderick GA, Kadioglu A, Bivalacqua TJ, Ghanem H, Nehra A, Shamloul R (January 2010). "Priapism: pathogenesis, epidemiology, and management". The Journal of Sexual Medicine. 7 (1 Pt 2): 476–500. doi:10.1111/j.1743-6109.2009.01625.x. PMID 20092449.
- ^ Chow K, Payne S (December 2008). "The pharmacological management of intermittent priapismic states". BJU International. 102 (11): 1515–1521. doi:10.1111/j.1464-410X.2008.07951.x. PMID 18793304. S2CID 35399393.
- ^ Dahm P, Rao DS, Donatucci CF (January 2002). "Antiandrogens in the treatment of priapism". Urology. 59 (1): 138. doi:10.1016/S0090-4295(01)01492-3. PMID 11796309.
- ^ Gooren LJ (2011). "Clinical review: Ethical and medical considerations of androgen deprivation treatment of sex offenders". The Journal of Clinical Endocrinology & Metabolism. 96 (12): 3628–37. doi:10.1210/jc.2011-1540. PMID 21956411.
- ^ Giltay EJ, Gooren LJ (2009). "Potential side effects of androgen deprivation treatment in sex offenders". The Journal of the American Academy of Psychiatry and the Law. 37 (1): 53–8. PMID 19297634.
- ^ Khan O, Mashru A (2016). "The efficacy, safety and ethics of the use of testosterone-suppressing agents in the management of sex offending". Current Opinion in Endocrinology, Diabetes and Obesity. 23 (3): 271–8. doi:10.1097/MED.0000000000000257. PMID 27032060. S2CID 43286710.
- ^ Dangerous Sex Offenders: A Task Force Report of the American Psychiatric Association. American Psychiatric Pub. 1999. pp. 111–. ISBN 978-0-89042-280-9.
- ^ Houts FW, Taller I, Tucker DE, Berlin FS (2011). "Androgen deprivation treatment of sexual behavior". Advances in Psychosomatic Medicine. 31: 149–63. doi:10.1159/000330196. ISBN 978-3-8055-9825-5. PMID 22005210.
- ^ Rousseau L, Couture M, Dupont A, Labrie F, Couture N (1990). "Effect of combined androgen blockade with an LHRH agonist and flutamide in one severe case of male exhibitionism". The Canadian Journal of Psychiatry. 35 (4): 338–41. doi:10.1177/070674379003500412. PMID 2189544. S2CID 28970865.
- ^ a b c d e Swiss Pharmaceutical Society, ed. (January 2000). Index Nominum 2000: International Drug Directory. Taylor & Francis. pp. 123–. ISBN 978-3-88763-075-1. Archived from the original on 24 April 2016.
- ^ a b c Sweetman SC (2011). Martindale: The Complete Drug Reference. Pharmaceutical Press. pp. 750–751. ISBN 978-0-85369-933-0. Archived from the original on 14 January 2023. Retrieved 27 September 2016.
- ^ a b White R, Bradnam V (11 March 2015). Handbook of Drug Administration via Enteral Feeding Tubes (3rd ed.). Pharmaceutical Press. pp. 133–. ISBN 978-0-85711-162-3. Archived from the original on 14 January 2023. Retrieved 27 September 2016.
- ^ a b Morton IK, Hall J (2001). The Avery Complete Guide to Medicines. Avery. pp. 105–106. ISBN 978-1-58333-105-7.
- ^ a b c d e Chabner BA, Longo DL (8 November 2010). Cancer Chemotherapy and Biotherapy: Principles and Practice. Lippincott Williams & Wilkins. pp. 679–680. ISBN 978-1-60547-431-1. Archived from the original on 10 January 2023. Retrieved 27 September 2016.
From a structural standpoint, antiandrogens are classified as steroidal, including cyproterone [acetate] (Androcur) and megestrol [acetate], or nonsteroidal, including flutamide (Eulexin, others), bicalutamide (Casodex), and nilutamide (Nilandron). The steroidal antiandrogens are rarely used.
- ^ Kolvenbag GJ, Furr BJ, Blackledge GR (December 1998). "Receptor affinity and potency of non-steroidal antiandrogens: translation of preclinical findings into clinical activity". Prostate Cancer Prostatic Dis. 1 (6): 307–314. doi:10.1038/sj.pcan.4500262. PMID 12496872. S2CID 33497597.
In addition, since bicalutamide has a low solubility, authentic Casodex® is micronised to ensure a small and consistent particle size to optimise bioavailability.
- ^ a b "Zolacos CP". Drugs.com. Archived from the original on 20 September 2016.
- ^ a b "Zolacos CP" (PDF). MIMS/myDr. April 2007. Archived from the original (PDF) on 17 September 2016.
- ^ a b "ZOLACOS CP" (PDF). New Zealand Data Sheet. 25 July 2016. Archived (PDF) from the original on 19 September 2016.
- ^ a b c d e f g h "COSUDEX® (bicalutamide) 150 mg tablets". TGA. Archived from the original on 14 September 2016.
- ^ a b c Iswaran TJ, Imai M, Betton GR, Siddall RA (May 1997). "An overview of animal toxicology studies with bicalutamide (ICI 176,334)". The Journal of Toxicological Sciences. 22 (2): 75–88. doi:10.2131/jts.22.2_75. PMID 9198005.
- ^ a b Smith RE (4 April 2013). Medicinal Chemistry – Fusion of Traditional and Western Medicine. Bentham Science Publishers. pp. 306–. ISBN 978-1-60805-149-6. Archived from the original on 29 May 2016.
- ^ Skeel RT, Khleif SN (2011). Handbook of Cancer Chemotherapy. Lippincott Williams & Wilkins. pp. 724–. ISBN 9781608317820. Archived from the original on 29 May 2016.
- ^ Mosby's GenRx: A Comprehensive Reference for Generic and Brand Prescription Drugs. Mosby. 2001. pp. 289–290. ISBN 978-0-323-00629-3.
- ^ Duplay D (2004). Physicians' Desk Reference. Thomson PDR. ISBN 978-1-56363-471-0. Archived from the original on 14 January 2023. Retrieved 27 September 2016.
- ^ Mcleod DG (September 2002). "Emerging role of adjuvant hormonal therapy". Urology. 60 (3 Suppl 1): 13–20, discussion 21. doi:10.1016/S0090-4295(02)01562-5. PMID 12231039.
- ^ "Bicalutamide" (PDF). Richmond Hill, Ontario: Nu-Pharm Inc. October 2009.
- ^ Bennett CL, Raisch DW, Sartor O (October 2002). "Pneumonitis associated with nonsteroidal antiandrogens: presumptive evidence of a class effect". Annals of Internal Medicine. 137 (7): 625. doi:10.7326/0003-4819-137-7-200210010-00029. PMID 12353966.
An estimated 0.77% of the 6,480 nilutamide-treated patients, 0.04% of the 41,700 flutamide-treated patients, and 0.01% of the 86,800 bicalutamide-treated patients developed pneumonitis during the study period.
- ^ Molina Mancero G, Picón X, Di Tullio F, Ernst G, Dezanzo P, Salvado A, Chertcoff JF (October 2016). "Neumonía intersticial inducida por bloqueo androgénico máximo como tratamiento de cáncer de próstata avanzado" [Fatal interstitial lung disease associated with maximum androgen blockade. Report of one case]. Revista médica de Chile (in Spanish). 144 (10): 1356–1359. doi:10.4067/S0034-98872016001000017. PMID 28074993.
- ^ Lee K, Oda Y, Sakaguchi M, Yamamoto A, Nishigori C (May 2016). "Drug-induced photosensitivity to bicalutamide - case report and review of the literature". Photodermatology, Photoimmunology & Photomedicine. 32 (3): 161–164. doi:10.1111/phpp.12230. PMID 26663090. S2CID 2761388.
- ^ Gretarsdottir HM, Bjornsdottir E, Bjornsson ES (2018). "Bicalutamide-Associated Acute Liver Injury and Migratory Arthralgia: A Rare but Clinically Important Adverse Effect". Case Reports in Gastroenterology. 12 (2): 266–270. doi:10.1159/000485175. ISSN 1662-0631. S2CID 81661015.
- ^ Anderson J (March 2003). "The role of antiandrogen monotherapy in the treatment of prostate cancer". BJU International. 91 (5): 455–461. doi:10.1046/j.1464-410X.2003.04026.x. PMID 12603397. S2CID 8639102.
- ^ a b c d Lehne RA (2013). Pharmacology for Nursing Care. Elsevier Health Sciences. pp. 1297–. ISBN 978-1-4377-3582-6. Archived from the original on 10 January 2023. Retrieved 27 September 2016.
- ^ a b c d Wirth MP, Hakenberg OW, Froehner M (February 2007). "Antiandrogens in the treatment of prostate cancer". European Urology. 51 (2): 306–13, discussion 314. doi:10.1016/j.eururo.2006.08.043. PMID 17007995.
- ^ a b c d e f Wellington K, Keam SJ (2006). "Bicalutamide 150mg: a review of its use in the treatment of locally advanced prostate cancer". Drugs. 66 (6): 837–50. doi:10.2165/00003495-200666060-00007. PMID 16706554. S2CID 46966712.
- ^ Higano CS (February 2003). "Side effects of androgen deprivation therapy: monitoring and minimizing toxicity". Urology. 61 (2 Suppl 1): 32–8. doi:10.1016/S0090-4295(02)02397-X. PMID 12667885.
- ^ Higano CS (2012). "Sexuality and intimacy after definitive treatment and subsequent androgen deprivation therapy for prostate cancer". Journal of Clinical Oncology. 30 (30): 3720–5. doi:10.1200/JCO.2012.41.8509. PMID 23008326.
- ^ a b c d e f g h Kolvenbag GJ, Blackledge GR (January 1996). "Worldwide activity and safety of bicalutamide: a summary review". Urology. 47 (1A Suppl): 70–9, discussion 80–4. doi:10.1016/s0090-4295(96)80012-4. PMID 8560681.
Bicalutamide is a new antiandrogen that offers the convenience of once-daily administration, demonstrated activity in prostate cancer, and an excellent safety profile. Because it is effective and offers better tolerability than flutamide, bicalutamide represents a valid first choice for antiandrogen therapy in combination with castration for the treatment of patients with advanced prostate cancer.
- ^ Resnick MI, Thompson IM (2000). Advanced Therapy of Prostate Disease. PMPH-USA. pp. 379–. ISBN 978-1-55009-102-1. Archived from the original on 10 June 2016.
- ^ a b c d e Iversen P, Melezinek I, Schmidt A (January 2001). "Nonsteroidal antiandrogens: a therapeutic option for patients with advanced prostate cancer who wish to retain sexual interest and function". BJU International. 87 (1): 47–56. doi:10.1046/j.1464-410x.2001.00988.x. PMID 11121992. S2CID 28215804.
- ^ a b c d e f g Anderson J (March 2003). "The role of antiandrogen monotherapy in the treatment of prostate cancer". BJU Int. 91 (5): 455–61. doi:10.1046/j.1464-410X.2003.04026.x. PMID 12603397. S2CID 8639102.
- ^ Kathryn Korkidakis A, Reid RL (2017). "Testosterone in Women: Measurement and Therapeutic Use". Journal of Obstetrics and Gynaecology Canada. 39 (3): 124–130. doi:10.1016/j.jogc.2017.01.006. PMID 28343552.
- ^ Davis SR, Wahlin-Jacobsen S (2015). "Testosterone in women--the clinical significance". The Lancet Diabetes & Endocrinology. 3 (12): 980–92. doi:10.1016/S2213-8587(15)00284-3. PMID 26358173.
- ^ Cignarella A, Mioni R, Sabbadin C, Dassie F, Parolin M, Vettor R, Barbot M, Scaroni C (December 2020). "Pharmacological Approaches to Controlling Cardiometabolic Risk in Women with PCOS". Int J Mol Sci. 21 (24): 9554. doi:10.3390/ijms21249554. PMC 7765466. PMID 33334002.
- ^ Luque-Ramírez M, Ortiz-Flores AE, Nattero-Chávez L, Escobar-Morreale HF (December 2020). "A safety evaluation of current medications for adult women with the polycystic ovarian syndrome not pursuing pregnancy". Expert Opin Drug Saf. 19 (12): 1559–1576. doi:10.1080/14740338.2020.1839409. PMID 33070640. S2CID 224784192.
- ^ Fourcade RO, McLeod D (March 2004). "Tolerability of Antiandrogens in the Treatment of Prostate Cancer". UroOncology. 4 (1): 5–13. doi:10.1080/1561095042000191655. ISSN 1561-0950.
Based on the available evidence, bicalutamide appears to have a better profile of non-pharmacological side effects than either flutamide or nilutamide; no specific nonpharmacological complications have yet been linked to this agent, while the incidence of the side effects such as diarrhoea and abnormal liver function appears to be lower than for the other two non-steroidal compounds. Furthermore, the recent data from the EPC programme suggest that the non-pharmacological side-effect profile of bicalutamide is not dissimilar to that of placebo (Table m [3].
- ^ Lunglmayr G (August 1995). "Efficacy and tolerability of Casodex in patients with advanced prostate cancer. International Casodex Study Group". Anti-Cancer Drugs. 6 (4): 508–13. doi:10.1097/00001813-199508000-00003. PMID 7579554.
- ^ McLeod DG (1997). "Tolerability of Nonsteroidal Antiandrogens in the Treatment of Advanced Prostate Cancer". Oncologist. 2 (1): 18–27. doi:10.1634/theoncologist.2-1-18. PMID 10388026.
- ^ DeAngelis LM, Posner JB (12 September 2008). Neurologic Complications of Cancer. Oxford University Press, USA. pp. 479–. ISBN 978-0-19-971055-3. Archived from the original on 7 May 2016.
- ^ Jamnicky L, Nam R (5 November 2012). Canadian Guide to Prostate Cancer. John Wiley & Sons. pp. 177–. ISBN 978-1-118-51565-5. Archived from the original on 14 January 2023. Retrieved 27 September 2016.
- ^ Tyrrell CJ, Denis L, Newling D, Soloway M, Channer K, Cockshott ID (1998). "Casodex 10-200 mg daily, used as monotherapy for the treatment of patients with advanced prostate cancer. An overview of the efficacy, tolerability and pharmacokinetics from three phase II dose-ranging studies. Casodex Study Group". Eur. Urol. 33 (1): 39–53. doi:10.1159/000019526. PMID 9471040. S2CID 71758492. Archived from the original on 1 February 2022. Retrieved 13 April 2023.
- ^ Tyrrell CJ, Iversen P, Tammela T, Anderson J, Björk T, Kaisary AV, Morris T (September 2006). "Tolerability, efficacy and pharmacokinetics of bicalutamide 300 mg, 450 mg or 600 mg as monotherapy for patients with locally advanced or metastatic prostate cancer, compared with castration". BJU International. 98 (3): 563–72. doi:10.1111/j.1464-410X.2006.06275.x. PMID 16771791. S2CID 41672303.
- ^ a b See WA, Wirth MP, McLeod DG, Iversen P, Klimberg I, Gleason D, et al. (August 2002). "Bicalutamide as immediate therapy either alone or as adjuvant to standard care of patients with localized or locally advanced prostate cancer: first analysis of the early prostate cancer program". The Journal of Urology. 168 (2): 429–35. doi:10.1016/S0022-5347(05)64652-6. PMID 12131282.
- ^ Schellhammer P, Sharifi R, Block N, Soloway M, Venner P, Patterson AL, Sarosdy M, Vogelzang N, Jones J, Kolvenbag G (January 1996). "Maximal androgen blockade for patients with metastatic prostate cancer: outcome of a controlled trial of bicalutamide versus flutamide, each in combination with luteinizing hormone-releasing hormone analogue therapy. Casodex Combination Study Group". Urology. 47 (1A Suppl): 54–60, discussion 80–4. doi:10.1016/s0090-4295(96)80010-0. PMID 8560679.
- ^ Iversen P, Johansson JE, Lodding P, Lukkarinen O, Lundmo P, Klarskov P, Tammela TL, Tasdemir I, Morris T, Carroll K (November 2004). "Bicalutamide (150 mg) versus placebo as immediate therapy alone or as adjuvant to therapy with curative intent for early nonmetastatic prostate cancer: 5.3-year median followup from the Scandinavian Prostate Cancer Group Study Number 6". The Journal of Urology. 172 (5 Pt 1): 1871–6. doi:10.1097/01.ju.0000139719.99825.54. PMID 15540741.
- ^ Wellington K, Keam SJ (2006). "Bicalutamide 150mg: a review of its use in the treatment of locally advanced prostate cancer". Drugs. 66 (6): 837–50. doi:10.2165/00003495-200666060-00007. PMID 16706554. S2CID 46966712.
- ^ Iversen P, Johansson JE, Lodding P, Kylmälä T, Lundmo P, Klarskov P, Tammela TL, Tasdemir I, Morris T, Armstrong J (2006). "Bicalutamide 150 mg in addition to standard care for patients with early non-metastatic prostate cancer: updated results from the Scandinavian Prostate Cancer Period Group-6 Study after a median follow-up period of 7.1 years". Scandinavian Journal of Urology and Nephrology. 40 (6): 441–52. doi:10.1080/00365590601017329. PMID 17130095. S2CID 25862814.
- ^ a b Trüeb RM, Luu NC, Uribe NC, Régnier A (December 2022). "Comment on: Bicalutamide and the new perspectives for female pattern hair loss treatment: What dermatologists should know". J Cosmet Dermatol. 21 (12): 7200–7201. doi:10.1111/jocd.14936. PMID 35332669. S2CID 247677549.
Indeed, due to the minimal biological importance of androgens in women, the adverse effects of bicalutamide are few. And yet, bicalutamide has been associated with elevated liver enzymes, and as of 2021, there have been 10 case reports of liver toxicity associated with bicalutamide, with fatality occurring in 2 cases.2
- ^ a b Gretarsdottir HM, Bjornsdottir E, Bjornsson ES (2018). "Bicalutamide-Associated Acute Liver Injury and Migratory Arthralgia: A Rare but Clinically Important Adverse Effect". Case Reports in Gastroenterology. 12 (2): 266–70. doi:10.1159/000485175. hdl:20.500.11815/1492. ISSN 1662-0631.
- ^ a b c Hussain S, Haidar A, Bloom RE, Zayouna N, Piper MH, Jafri SM (2014). "Bicalutamide-induced hepatotoxicity: A rare adverse effect". Am J Case Rep. 15: 266–70. doi:10.12659/AJCR.890679. PMC 4068966. PMID 24967002.
- ^ a b Yun GY, Kim SH, Kim SW, Joo JS, Kim JS, Lee ES, Lee BS, Kang SH, Moon HS, Sung JK, Lee HY, Kim KH (April 2016). "Atypical onset of bicalutamide-induced liver injury". World J. Gastroenterol. 22 (15): 4062–5. doi:10.3748/wjg.v22.i15.4062. PMC 4823258. PMID 27099451.
- ^ a b O'Bryant CL, Flaig TW, Utz KJ (2008). "Bicalutamide-associated fulminant hepatotoxicity". Pharmacotherapy. 28 (8): 1071–5. doi:10.1592/phco.28.8.1071. PMID 18657023. S2CID 20315801.
- ^ Castro Beza I, Sánchez Ruiz J, Peracaula Espino FJ, Villanego Beltrán MI (September 2008). "Drug-related hepatotoxicity and hepatic failure following combined androgen blockade". Clin Transl Oncol. 10 (9): 591–2. doi:10.1007/s12094-008-0256-5. PMID 18796378.
- ^ a b "FDA Adverse Event Reporting System (FAERS) Public Dashboard". FDA. 22 October 2021. Archived from the original on 15 February 2023. Retrieved 15 February 2023.
- ^ Dart RC (2004). Medical Toxicology. Lippincott Williams & Wilkins. pp. 497–. ISBN 978-0-7817-2845-4. Archived from the original on 11 May 2016.
- ^ Masago T, Watanabe T, Nemoto R, Motoda K (December 2011). "Interstitial pneumonitis induced by bicalutamide given for prostate cancer". International Journal of Clinical Oncology. 16 (6): 763–5. doi:10.1007/s10147-011-0239-x. PMID 21537882. S2CID 24068787.
- ^ Aronson JK (4 March 2014). Side Effects of Drugs Annual: A worldwide yearly survey of new data in adverse drug reactions. Newnes. pp. 740–. ISBN 978-0-444-62636-3. Archived from the original on 6 May 2016.
- ^ Daba MH, El-Tahir KE, Al-Arifi MN, Gubara OA (June 2004). "Drug-induced pulmonary fibrosis". Saudi Medical Journal. 25 (6): 700–6. PMID 15195196.
- ^ Thole Z, Manso G, Salgueiro E, Revuelta P, Hidalgo A (2004). "Hepatotoxicity induced by antiandrogens: a review of the literature". Urologia Internationalis. 73 (4): 289–95. doi:10.1159/000081585. PMID 15604569. S2CID 24799765.
- ^ Ricci F, Buzzatti G, Rubagotti A, Boccardo F (November 2014). "Safety of antiandrogen therapy for treating prostate cancer". Expert Opinion on Drug Safety. 13 (11): 1483–99. doi:10.1517/14740338.2014.966686. PMID 25270521. S2CID 207488100.
- ^ Sex Differences in the Human Brain, their underpinnings and implications. Elsevier. 3 December 2010. pp. 44–45. ISBN 978-0-444-53631-0. Archived from the original on 26 May 2016.
- ^ Paoletti R (6 December 2012). Chemistry and Brain Development: Proceedings of the Advanced Study Institute on "Chemistry of Brain Development," held in Milan, Italy, September 9–19, 1970. Springer Science & Business Media. pp. 218–. ISBN 978-1-4684-7236-3. Archived from the original on 14 January 2023. Retrieved 27 September 2016.
- ^ Papadimitriou K, Anagnostis P, Goulis DG (2022). "The challenging role of antiandrogens in the management of polycystic ovary syndrome". Polycystic Ovary Syndrome. Elsevier. pp. 297–314. doi:10.1016/B978-0-12-823045-9.00013-4. ISBN 9780128230459. S2CID 244697776.
- ^ Ramon J, Denis LJ (5 June 2007). Prostate Cancer. Springer Science & Business Media. pp. 256–. ISBN 978-3-540-40901-4.
- ^ Moser L (1 January 2008). Controversies in the Treatment of Prostate Cancer. Karger Medical and Scientific Publishers. pp. 41–. ISBN 978-3-8055-8524-8. Archived from the original on 12 January 2023. Retrieved 5 January 2016.
- ^ Prostate Cancer. Demos Medical Publishing. 20 December 2011. pp. 504–505. ISBN 978-1-935281-91-7. Archived from the original on 14 January 2023. Retrieved 21 December 2017.
- ^ a b c d Aronson JK (21 February 2009). Meyler's Side Effects of Endocrine and Metabolic Drugs. Elsevier. pp. 149–150, 253–258. ISBN 978-0-08-093292-7. Archived from the original on 14 January 2023. Retrieved 21 December 2017.
- ^ Barrett J (2007). Transsexual and Other Disorders of Gender Identity: A Practical Guide to Management. Radcliffe Publishing. pp. 174–. ISBN 978-1-85775-719-4. Archived from the original on 14 January 2023. Retrieved 27 September 2016.
- ^ Rushton DH (2002). "Nutritional factors and hair loss". Clin. Exp. Dermatol. 27 (5): 396–404. doi:10.1046/j.1365-2230.2002.01076.x. PMID 12190640. S2CID 39327815.
- ^ Boccardo F (2000). "Hormone therapy of prostate cancer: is there a role for antiandrogen monotherapy?". Crit. Rev. Oncol. Hematol. 35 (2): 121–32. doi:10.1016/s1040-8428(00)00051-2. PMID 10936469.
- ^ Thole Z, Manso G, Salgueiro E, Revuelta P, Hidalgo A (2004). "Hepatotoxicity induced by antiandrogens: a review of the literature". Urol. Int. 73 (4): 289–95. doi:10.1159/000081585. PMID 15604569. S2CID 24799765.
- ^ a b Craig JV, Furr BJ (5 February 2010). Hormone Therapy in Breast and Prostate Cancer. Springer Science & Business Media. pp. 356–. ISBN 978-1-59259-152-7. Archived from the original on 14 January 2023. Retrieved 27 September 2016.
- ^ Bennett CL, Raisch DW, Sartor O (October 2002). "Pneumonitis associated with nonsteroidal antiandrogens: presumptive evidence of a class effect". Annals of Internal Medicine. 137 (7): 625. doi:10.7326/0003-4819-137-7-200210010-00029. PMID 12353966.
An estimated 0.77% of the 6,480 nilutamide-treated patients, 0.04% of the 41,700 flutamide-treated patients, and 0.01% of the 86,800 bicalutamide-treated patients developed pneumonitis during the study period.
- ^ Ricci F, Buzzatti G, Rubagotti A, Boccardo F (2014). "Safety of antiandrogen therapy for treating prostate cancer". Expert Opin Drug Saf. 13 (11): 1483–99. doi:10.1517/14740338.2014.966686. PMID 25270521. S2CID 207488100.
- ^ Foster WR, Car BD, Shi H, Levesque PC, Obermeier MT, Gan J, Arezzo JC, Powlin SS, Dinchuk JE, Balog A, Salvati ME, Attar RM, Gottardis MM (2011). "Drug safety is a barrier to the discovery and development of new androgen receptor antagonists". Prostate. 71 (5): 480–8. doi:10.1002/pros.21263. PMID 20878947. S2CID 24620044.
- ^ Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S, Carles J, Chowdhury S, Davis ID, de Bono JS, Evans CP, Fizazi K, Joshua AM, Kim CS, Kimura G, Mainwaring P, Mansbach H, Miller K, Noonberg SB, Perabo F, Phung D, Saad F, Scher HI, Taplin ME, Venner PM, Tombal B (2014). "Enzalutamide in metastatic prostate cancer before chemotherapy". N. Engl. J. Med. 371 (5): 424–33. doi:10.1056/NEJMoa1405095. PMC 4418931. PMID 24881730.
- ^ Keating GM (2015). "Enzalutamide: a review of its use in chemotherapy-naïve metastatic castration-resistant prostate cancer". Drugs Aging. 32 (3): 243–9. doi:10.1007/s40266-015-0248-y. PMID 25711765. S2CID 29563345.
- ^ Beer TM, Tombal B (2014). "Enzalutamide in metastatic prostate cancer before chemotherapy" (PDF). N. Engl. J. Med. 371 (18): 1755–6. doi:10.1056/NEJMc1410239. hdl:2318/150443. PMID 25354111. Archived (PDF) from the original on 28 August 2021. Retrieved 23 September 2019.
- ^ Furr BJ, Tucker H (1996). "The preclinical development of bicalutamide: pharmacodynamics and mechanism of action". Urology. 47 (1A Suppl): 13–25, discussion 29–32. doi:10.1016/S0090-4295(96)80003-3. PMID 8560673.
- ^ Lenz AM, Shulman D, Eugster EA, Rahhal S, Fuqua JS, Pescovitz OH, Lewis KA (September 2010). "Bicalutamide and third-generation aromatase inhibitors in testotoxicosis". Pediatrics. 126 (3): e728-33. doi:10.1542/peds.2010-0596. PMC 4096839. PMID 20713483.
- ^ Greenblatt DJ, Koch-Weser J (July 1973). "Adverse reactions to spironolactone. A report from the Boston Collaborative Drug Surveillance Program". JAMA. 225 (1): 40–3. doi:10.1001/jama.1973.03220280028007. PMID 4740303.
- ^ Munoz R, da Cruz E, Vetterly CG, Cooper D, Berry D (26 June 2014). Handbook of Pediatric Cardiovascular Drugs. Springer. pp. 224–. ISBN 978-1-4471-2464-1. Archived from the original on 14 January 2023. Retrieved 27 September 2016.
- ^ Bahceci M, Tuzcu A, Canoruc N, Tuzun Y, Kidir V, Aslan C (2004). "Serum C-reactive protein (CRP) levels and insulin resistance in non-obese women with polycystic ovarian syndrome, and effect of bicalutamide on hirsutism, CRP levels and insulin resistance". Hormone Research. 62 (6): 283–7. doi:10.1159/000081973. PMID 15542929. S2CID 46261843.
- ^ a b Nurse Practitioner's Drug Handbook. Springhouse Corp. 2000. ISBN 9780874349979. Archived from the original on 14 January 2023. Retrieved 27 September 2016.
- ^ a b c Tyrrell CJ, Iversen P, Tammela T, Anderson J, Björk T, Kaisary AV, Morris T (September 2006). "Tolerability, efficacy and pharmacokinetics of bicalutamide 300 mg, 450 mg or 600 mg as monotherapy for patients with locally advanced or metastatic prostate cancer, compared with castration". BJU International. 98 (3): 563–72. doi:10.1111/j.1464-410X.2006.06275.x. PMID 16771791. S2CID 41672303.
- ^ Griffith HW (2008). Complete Guide to Prescription & Nonprescription Drugs 2009. HP Books. pp. 62–. ISBN 978-0-399-53463-8.
Overdose unlikely to threaten life [with NSAAs].
- ^ Genrx (1999). 1999 Mosby's GenRx. Mosby. ISBN 978-0-323-00625-5.
A 79-year-old man attempted suicide by ingesting 13g of nilutamide (i.e., 43 times the maximum recommended dose). Despite immediate gastric lavage and oral administration of activated charcoal, plasma nilutamide levels peaked at 6 times the normal range 2 hours after ingestion. There were no clinical signs or symptoms or changes in parameters such as transaminases or chest x-ray. Maintenance treatment (150 mg/day) was resumed 30 days later.
- ^ a b c d e f g h i Weber GF (22 July 2015). Molecular Therapies of Cancer. Springer. pp. 318–. ISBN 978-3-319-13278-5.
Compared to flutamide and nilutamide, bicalutamide has a 2-fold increased affinity for the Androgen Receptor, a longer half-life, and substantially reduced toxicities. Based on a more favorable safety profile relative to flutamide, bicalutamide is indicated for use in combination therapy with a Gonadotropin Releasing Hormone analog for the treatment of advanced metastatic prostate carcinoma.
- ^ Mosby's GenRx: A Comprehensive Reference for Generic and Brand Prescription Drugs. Mosby. 2001. p. 290. ISBN 978-0-323-00629-3.
In vitro studies have shown bicalutamide can displace coumarin anticoagulants, such as warfarin, from their protein-binding sites. It is recommended that if bicalutamide is started in patients already receiving coumarin anticoagulants, prothrombin times should be closely monitored and adjustment of the anticoagulant dose may be necessary.
- ^ Spratto G, Woods A (2 July 2008). 2009 Edition Delmar's Nurse's Drug Handbook. Cengage Learning. pp. 175–. ISBN 978-1-4283-6106-5. Archived from the original on 14 January 2023. Retrieved 27 September 2016.
- ^ Balaj KC (25 April 2016). Managing Metastatic Prostate Cancer In Your Urological Oncology Practice. Springer. pp. 24–25. ISBN 978-3-319-31341-2. Archived from the original on 8 September 2017.
- ^ Masiello D, Cheng S, Bubley GJ, Lu ML, Balk SP (July 2002). "Bicalutamide functions as an androgen receptor antagonist by assembly of a transcriptionally inactive receptor". The Journal of Biological Chemistry. 277 (29): 26321–6. doi:10.1074/jbc.M203310200. PMID 12015321.
- ^ a b c d Denis L (6 December 2012). Antiandrogens in Prostate Cancer: A Key to Tailored Endocrine Treatment. Springer Science & Business Media. pp. 128, 158, 203. ISBN 978-3-642-45745-6. Archived from the original on 14 January 2023. Retrieved 27 September 2016.
- ^ a b c d Schellens JH, McLeod HL, Newell DR (5 May 2005). Cancer Clinical Pharmacology. OUP Oxford. pp. 229–230. ISBN 978-0-19-262966-1. Archived from the original on 10 June 2016.
- ^ a b c Becker KL (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 1119, 1196, 1208. ISBN 978-0-7817-1750-2. Archived from the original on 8 September 2017.
- ^ Ito Y, Sadar MD (2018). "Enzalutamide and blocking androgen receptor in advanced prostate cancer: lessons learnt from the history of drug development of antiandrogens". Res Rep Urol. 10: 23–32. doi:10.2147/RRU.S157116. PMC 5818862. PMID 29497605.
- ^ a b c Furr BJ (June 1995). "Casodex: preclinical studies and controversies". Annals of the New York Academy of Sciences. 761 (1): 79–96. Bibcode:1995NYASA.761...79F. doi:10.1111/j.1749-6632.1995.tb31371.x. PMID 7625752. S2CID 37242269.
- ^ Guise TA, Oefelein MG, Eastham JA, Cookson MS, Higano CS, Smith MR (2007). "Estrogenic side effects of androgen deprivation therapy". Reviews in Urology. 9 (4): 163–80. PMC 2213888. PMID 18231613.
- ^ Bulldan A, Malviya VN, Upmanyu N, Konrad L, Scheiner-Bobis G (2017). "Testosterone/bicalutamide antagonism at the predicted extracellular androgen binding site of ZIP9". Biochim. Biophys. Acta. 1864 (12): 2402–2414. doi:10.1016/j.bbamcr.2017.09.012. PMID 28943399.
- ^ Pi M, Parrill AL, Quarles LD (2010). "GPRC6A mediates the non-genomic effects of steroids". J. Biol. Chem. 285 (51): 39953–64. doi:10.1074/jbc.M110.158063. PMC 3000977. PMID 20947496.
- ^ Furr BJ (2009). "Research on reproductive medicine in the pharmaceutical industry". Human Fertility. 1 (1): 56–63. doi:10.1080/1464727982000198131. PMID 11844311.
- ^ Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, Wasielewska T, Welsbie D, Chen CD, Higano CS, Beer TM, Hung DT, Scher HI, Jung ME, Sawyers CL (2009). "Development of a second-generation antiandrogen for treatment of advanced prostate cancer". Science. 324 (5928): 787–90. Bibcode:2009Sci...324..787T. doi:10.1126/science.1168175. PMC 2981508. PMID 19359544.
[...] bicalutamide has relatively low affinity for AR (at least 30-fold reduced relative to the natural ligand dihydrotestosterone (DHT)) (7), [...]
- ^ Furr BK (1997). "Relative potencies of flutamide and 'Casodex'". Endocrine-Related Cancer. 4 (2): 197–202. doi:10.1677/erc.0.0040197 (inactive 1 November 2024). ISSN 1351-0088.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Figg W, Chau CH, Small EJ (14 September 2010). Drug Management of Prostate Cancer. Springer Science & Business Media. pp. 56, 71–72, 75, 93. ISBN 978-1-60327-829-4. Archived from the original on 14 January 2023. Retrieved 27 September 2016.
- ^ Furr BJ (1996). "The development of Casodex (bicalutamide): preclinical studies". European Urology. 29 (Suppl 2): 83–95. doi:10.1159/000473846. PMID 8717469.
- ^ a b c d Denis L, Mahler C (January 1996). "Pharmacodynamics and pharmacokinetics of bicalutamide: defining an active dosing regimen". Urology. 47 (1A Suppl): 26–8, discussion 29–32. doi:10.1016/S0090-4295(96)80004-5. PMID 8560674.
- ^ Boccardo F, Rubagotti A, Conti G, Potenzoni D, Manganelli A, Del Monaco D (2005). "Exploratory study of drug plasma levels during bicalutamide 150 mg therapy co-administered with tamoxifen or anastrozole for prophylaxis of gynecomastia and breast pain in men with prostate cancer". Cancer Chemotherapy and Pharmacology. 56 (4): 415–20. doi:10.1007/s00280-005-1016-1. PMID 15838655. S2CID 23014567. Archived from the original on 28 August 2021. Retrieved 11 December 2019.
- ^ Luo S, Martel C, Chen C, Labrie C, Candas B, Singh SM, Labrie F (December 1997). "Daily dosing with flutamide or Casodex exerts maximal antiandrogenic activity". Urology. 50 (6): 913–9. doi:10.1016/S0090-4295(97)00393-2. PMID 9426723.
- ^ Melmed S, Polonsky KS, Reed Larsen P, Kronenberg HM (30 November 2015). Williams Textbook of Endocrinology. Elsevier Health Sciences. pp. 704–708, 711, 1104. ISBN 978-0-323-29738-7.
- ^ Moretti C, Guccione L, Di Giacinto P, Simonelli I, Exacoustos C, Toscano V, et al. (April 2016). Efficacy and Safety of Myo-Inositol Supplementation in the Treatment of Obese Hirsute PCOS Women: Comparative Evaluation with OCP+Bicalutamide Therapy. ENDO 2016. Boston, Massachusetts. Archived from the original on 1 February 2020. Retrieved 1 February 2020.
- ^ Eri LM, Haug E, Tveter KJ (March 1995). "Effects on the endocrine system of long-term treatment with the non-steroidal anti-androgen Casodex in patients with benign prostatic hyperplasia". British Journal of Urology. 75 (3): 335–40. doi:10.1111/j.1464-410X.1995.tb07345.x. PMID 7537602.
- ^ Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA (25 August 2011). Campbell-Walsh Urology: Expert Consult Premium Edition: Enhanced Online Features and Print, 4-Volume Set. Elsevier Health Sciences. pp. 2938–2939, 2946. ISBN 978-1-4160-6911-9. Archived from the original on 5 May 2016.
- ^ a b Diamanti-Kandarakis E, Nestler JE, Pandas D, Pasquale R (21 December 2009). Insulin Resistance and Polycystic Ovarian Syndrome: Pathogenesis, Evaluation, and Treatment. Springer Science & Business Media. pp. 75–. ISBN 978-1-59745-310-3. Archived from the original on 19 May 2016.
- ^ a b Carrell DT, Peterson CW (23 March 2010). Reproductive Endocrinology and Infertility: Integrating Modern Clinical and Laboratory Practice. Springer Science & Business Media. pp. 163–. ISBN 978-1-4419-1436-1. Archived from the original on 4 July 2014.
- ^ a b Bouchard P, Caraty A (15 November 1993). GnRH, GnRH Analogs, Gonadotropins and Gonadal Peptides. CRC Press. pp. 455–456. ISBN 978-0-203-09205-7.
[...] when male levels of androgens are achieved in plasma, their effects on gonadotropin secretion are similar in women and men. [...] administration of flutamide in a group of normally-cycling women produced a clinical improvement of acne and hirsutism without any significant hormonal change. [...] All these data emphasize that physiological levels of androgens have no action on the regulation of gonadotropins in normal women. [...] Androgens do not directly play a role in gonadotropin regulation [in women].
- ^ Sieber PR (December 2007). "Treatment of bicalutamide-induced breast events". Expert Review of Anticancer Therapy. 7 (12): 1773–9. doi:10.1586/14737140.7.12.1773. PMID 18062751. S2CID 40410461.
- ^ Asscheman H, Gooren LJ, Peereboom-Wynia JD (1989). "Reduction in undesired sexual hair growth with anandron in male-to-female transsexuals—experiences with a novel androgen receptor blocker". Clinical and Experimental Dermatology. 14 (5): 361–3. doi:10.1111/j.1365-2230.1989.tb02585.x. PMID 2612040. S2CID 45303518.
- ^ Rao BR, de Voogt HJ, Geldof AA, Gooren LJ, Bouman FG (1988). "Merits and considerations in the use of anti-androgen". Journal of Steroid Biochemistry. 31 (4B): 731–7. doi:10.1016/0022-4731(88)90024-6. PMID 3143862.
- ^ a b Wibowo E, Schellhammer P, Wassersug RJ (January 2011). "Role of estrogen in normal male function: clinical implications for patients with prostate cancer on androgen deprivation therapy". The Journal of Urology. 185 (1): 17–23. doi:10.1016/j.juro.2010.08.094. PMID 21074215.
- ^ a b Motofei IG, Rowland DL, Popa F, Kreienkamp D, Paunica S (July 2011). "Preliminary study with bicalutamide in heterosexual and homosexual patients with prostate cancer: a possible implication of androgens in male homosexual arousal". BJU International. 108 (1): 110–5. doi:10.1111/j.1464-410X.2010.09764.x. PMID 20955264. S2CID 45482984.
- ^ a b Wibowo E, Wassersug RJ (September 2013). "The effect of estrogen on the sexual interest of castrated males: Implications to prostate cancer patients on androgen-deprivation therapy". Critical Reviews in Oncology/Hematology. 87 (3): 224–38. doi:10.1016/j.critrevonc.2013.01.006. PMID 23484454.
- ^ King SR (2008). "Emerging roles for neurosteroids in sexual behavior and function". Journal of Andrology. 29 (5): 524–33. doi:10.2164/jandrol.108.005660. PMID 18567641.
- ^ Morali G, Oropeza MV, Lemus AE, Perez-Palacios G (September 1994). "Mechanisms regulating male sexual behavior in the rat: role of 3 alpha- and 3 beta-androstanediols". Biology of Reproduction. 51 (3): 562–71. doi:10.1095/biolreprod51.3.562. PMID 7803627.
- ^ Sánchez Montoya EL, Hernández L, Barreto-Estrada JL, Ortiz JG, Jorge JC (November 2010). "The testosterone metabolite 3α-diol enhances female rat sexual motivation when infused in the nucleus accumbens shell". The Journal of Sexual Medicine. 7 (11): 3598–609. doi:10.1111/j.1743-6109.2010.01937.x. PMC 4360968. PMID 20646182.
- ^ Chedrese PJ (13 June 2009). Reproductive Endocrinology: A Molecular Approach. Springer Science & Business Media. pp. 233–. ISBN 978-0-387-88186-7. Archived from the original on 5 September 2017.
- ^ Frye CA, Edinger KL, Lephart ED, Walf AA (2010). "3alpha-androstanediol, but not testosterone, attenuates age-related decrements in cognitive, anxiety, and depressive behavior of male rats". Frontiers in Aging Neuroscience. 2: 15. doi:10.3389/fnagi.2010.00015. PMC 2874398. PMID 20552051.
- ^ Huang Q, Zhu H, Fischer DF, Zhou JN (June 2008). "An estrogenic effect of 5alpha-androstane-3beta, 17beta-diol on the behavioral response to stress and on CRH regulation". Neuropharmacology. 54 (8): 1233–8. doi:10.1016/j.neuropharm.2008.03.016. PMID 18457850. S2CID 9052079.
- ^ Frye CA, Koonce CJ, Edinger KL, Osborne DM, Walf AA (November 2008). "Androgens with activity at estrogen receptor beta have anxiolytic and cognitive-enhancing effects in male rats and mice". Hormones and Behavior. 54 (5): 726–34. doi:10.1016/j.yhbeh.2008.07.013. PMC 3623974. PMID 18775724.
- ^ a b c Bambury RM, Scher HI (June 2015). "Enzalutamide: Development from bench to bedside". Urologic Oncology. 33 (6): 280–8. doi:10.1016/j.urolonc.2014.12.017. PMID 25797385.
- ^ Bambury RM, Rathkopf DE (August 2016). "Novel and next-generation androgen receptor-directed therapies for prostate cancer: Beyond abiraterone and enzalutamide". Urologic Oncology. 34 (8): 348–55. doi:10.1016/j.urolonc.2015.05.025. PMID 26162486.
- ^ a b Pinto Á (February 2014). "Beyond abiraterone: new hormonal therapies for metastatic castration-resistant prostate cancer". Cancer Biology & Therapy. 15 (2): 149–55. doi:10.4161/cbt.26724. PMC 3928129. PMID 24100689.
- ^ Orentreich N, Durr NP (July 1974). "Proceedings: Mammogenesis in transsexuals". The Journal of Investigative Dermatology. 63 (1): 142–146. doi:10.1111/1523-1747.ep12678272. PMID 4365991.
- ^ Strauss III JF, Barbieri RL (13 September 2013). Yen and Jaffe's Reproductive Endocrinology. Elsevier Health Sciences. pp. 236–237. ISBN 978-1-4557-2758-2. Archived from the original on 14 January 2017.
- ^ Wilson CB, Nizet V, Maldonado Y, Klein JO, Remington JS (2015). Remington and Klein's Infectious Diseases of the Fetus and Newborn Infant. Elsevier Health Sciences. pp. 190–. ISBN 978-0-323-24147-2. Archived from the original on 14 January 2017.
- ^ a b Kanhai RC, Hage JJ, van Diest PJ, Bloemena E, Mulder JW (January 2000). "Short-term and long-term histologic effects of castration and estrogen treatment on breast tissue of 14 male-to-female transsexuals in comparison with two chemically castrated men". The American Journal of Surgical Pathology. 24 (1): 74–80. doi:10.1097/00000478-200001000-00009. PMID 10632490. S2CID 37752666.
- ^ a b Lawrence AA (2006). "Transgender Health Concerns". In Meyer IH, Northridge ME (eds.). The Health of Sexual Minorities. New York: Springer. p. 476. doi:10.1007/978-0-387-31334-4_19. ISBN 978-0-387-28871-0.
- ^ a b Rosen PP (2009). Rosen's Breast Pathology (3 ed.). Philadelphia: Lippincott Williams & Wilkins. pp. 31–. ISBN 978-0-7817-7137-5.
- ^ Mulhall JP (21 February 2013). Fertility Preservation in Male Cancer Patients. Cambridge University Press. pp. 84–. ISBN 978-1-139-61952-3. Archived from the original on 29 April 2016.
- ^ Morgante E, Gradini R, Realacci M, Sale P, D'Eramo G, Perrone GA, Cardillo MR, Petrangeli E, Russo M, Di Silverio F (March 2001). "Effects of long-term treatment with the anti-androgen bicalutamide on human testis: an ultrastructural and morphometric study". Histopathology. 38 (3): 195–201. doi:10.1046/j.1365-2559.2001.01077.x. hdl:11573/387981. PMID 11260298. S2CID 36892099.
- ^ a b Schill W, Comhaire FH, Hargreave TB (26 August 2006). Andrology for the Clinician. Springer Science & Business Media. pp. 76–. ISBN 978-3-540-33713-3. Archived from the original on 26 May 2016.
- ^ a b Nieschlag E, Behre HM (6 December 2012). Testosterone: Action – Deficiency – Substitution. Springer Science & Business Media. pp. 130, 276. ISBN 978-3-642-72185-4. Archived from the original on 14 January 2023. Retrieved 3 November 2016.
- ^ a b Cheng CY (24 October 2009). Molecular Mechanisms in Spermatogenesis. Springer Science & Business Media. pp. 258–. ISBN 978-0-387-09597-4. Archived from the original on 14 January 2023. Retrieved 27 September 2016.
- ^ Neumann F, Schenck B (1980). "Antiandrogens: Basic Concepts and Clinical Trials". Regulation of Male Fertility. Springer. pp. 93–106. doi:10.1007/978-94-009-8875-0_10. ISBN 978-94-009-8877-4.
- ^ a b Johnson LR (14 October 2003). Essential Medical Physiology. Academic Press. pp. 731–. ISBN 978-0-08-047270-6. Archived from the original on 15 February 2017.
- ^ a b Jones CA, Reiter L, Greenblatt E (2016). "Fertility preservation in transgender patients". International Journal of Transgenderism. 17 (2): 76–82. doi:10.1080/15532739.2016.1153992. ISSN 1553-2739. S2CID 58849546.
Traditionally, patients have been advised to cryopreserve sperm prior to starting cross-sex hormone therapy as there is a potential for a decline in sperm motility with high-dose estrogen therapy over time (Lubbert et al., 1992). However, this decline in fertility due to estrogen therapy is controversial due to limited studies.
- ^ a b Payne AH, Hardy MP (28 October 2007). The Leydig Cell in Health and Disease. Springer Science & Business Media. pp. 422–431. ISBN 978-1-59745-453-7.
Estrogens are highly efficient inhibitors of the hypothalamic-hypophyseal-testicular axis (212–214). Aside from their negative feedback action at the level of the hypothalamus and pituitary, direct inhibitory effects on the testis are likely (215,216). [...] The histology of the testes [with estrogen treatment] showed disorganization of the seminiferous tubules, vacuolization and absence of lumen, and compartmentalization of spermatogenesis.
- ^ a b Wakelin SH, Maibach HI, Archer CB (1 June 2002). Systemic Drug Treatment in Dermatology: A Handbook. CRC Press. pp. 32–. ISBN 978-1-84076-013-2. Archived from the original on 25 July 2014.
[Cyproterone acetate] inhibits spermatogenesis and produces reversible infertility (but is not a male contraceptive).
- ^ a b Neumann F (1994). "The antiandrogen cyproterone acetate: discovery, chemistry, basic pharmacology, clinical use and tool in basic research". Exp. Clin. Endocrinol. 102 (1): 1–32. doi:10.1055/s-0029-1211261. PMID 8005205.
Spermatogenesis is also androgen-dependent and is inhibited by CPA, meaning that patients treated with high doses of CPA are sterile (Figure 23). All the effects of CPA are fully reversible.
- ^ a b Salam MA (2003). Principles & Practice of Urology: A Comprehensive Text. Universal-Publishers. pp. 684–. ISBN 978-1-58112-412-5.
Estrogens act primarily through negative feedback at the hypothalamic-pituitary level to reduce LH secretion and testicular androgen synthesis. [...] Interestingly, if the treatment with estrogens is discontinued after 3 yr. of uninterrupted exposure, serum testosterone may remain at castration levels for up to another 3 yr. This prolonged suppression is thought to result from a direct effect of estrogens on the Leydig cells.
- ^ Mast N, Lin JB, Pikuleva IA (September 2015). "Marketed Drugs Can Inhibit Cytochrome P450 27A1, a Potential New Target for Breast Cancer Adjuvant Therapy". Molecular Pharmacology. 88 (3): 428–36. doi:10.1124/mol.115.099598. PMC 4551053. PMID 26082378.
- ^ Mast N, Zheng W, Stout CD, Pikuleva IA (February 2013). "Binding of a cyano- and fluoro-containing drug bicalutamide to cytochrome P450 46A1: unusual features and spectral response". The Journal of Biological Chemistry. 288 (7): 4613–24. doi:10.1074/jbc.M112.438754. PMC 3576067. PMID 23288837.
- ^ Zhu Y, Liu C, Armstrong C, Lou W, Sandher A, Gao AC (September 2015). "Antiandrogens Inhibit ABCB1 Efflux and ATPase Activity and Reverse Docetaxel Resistance in Advanced Prostate Cancer". Clinical Cancer Research. 21 (18): 4133–42. doi:10.1158/1078-0432.CCR-15-0269. PMC 4573793. PMID 25995342.
- ^ Fenner A (July 2015). "Prostate cancer: Antiandrogens reverse docetaxel resistance via ABCB1 inhibition". Nature Reviews. Urology. 12 (7): 361. doi:10.1038/nrurol.2015.135. PMID 26057062. S2CID 29729317.
- ^ Armstrong CM, Gao AC (2015). "Drug resistance in castration resistant prostate cancer: resistance mechanisms and emerging treatment strategies". American Journal of Clinical and Experimental Urology. 3 (2): 64–76. PMC 4539108. PMID 26309896.
- ^ a b Foster WR, Car BD, Shi H, Levesque PC, Obermeier MT, Gan J, Arezzo JC, Powlin SS, Dinchuk JE, Balog A, Salvati ME, Attar RM, Gottardis MM (April 2011). "Drug safety is a barrier to the discovery and development of new androgen receptor antagonists". The Prostate. 71 (5): 480–8. doi:10.1002/pros.21263. PMID 20878947. S2CID 24620044.
- ^ a b Barrish J, Carter P, Cheng P (2010). Accounts in Drug Discovery: Case Studies in Medicinal Chemistry. Royal Society of Chemistry. pp. 127–. ISBN 978-1-84973-126-3. Archived from the original on 14 January 2023. Retrieved 27 September 2016.
- ^ Kolvenbag GJ, Blackledge GR, Gotting-Smith K (January 1998). "Bicalutamide (Casodex) in the treatment of prostate cancer: history of clinical development". The Prostate. 34 (1): 61–72. doi:10.1002/(SICI)1097-0045(19980101)34:1<61::AID-PROS8>3.0.CO;2-N. PMID 9428389. S2CID 22785559.
- ^ Blackledge GR (1996). "Clinical progress with a new antiandrogen, Casodex (bicalutamide)". Eur. Urol. 29 (Suppl 2): 96–104. doi:10.1159/000473847. PMID 8717470.
- ^ DeVita Jr VT, Lawrence TS, Rosenberg SA (7 January 2015). DeVita, Hellman, and Rosenberg's Cancer: Principles & Practice of Oncology. Wolters Kluwer Health. pp. 1142–. ISBN 978-1-4698-9455-3.
- ^ a b Chu E, DeVita Jr VT (28 December 2012). Physicians' Cancer Chemotherapy Drug Manual 2013. Jones & Bartlett Publishers. pp. 51–. ISBN 978-1-284-04039-5. Archived from the original on 14 January 2023. Retrieved 27 September 2016.
- ^ a b Helsen C, Van den Broeck T, Voet A, Prekovic S, Van Poppel H, Joniau S, Claessens F (August 2014). "Androgen receptor antagonists for prostate cancer therapy". Endocrine-Related Cancer. 21 (4): T105-18. doi:10.1530/ERC-13-0545. PMID 24639562.
- ^ Furr BJ (1989). ""Casodex" (ICI 176,334)--a new, pure, peripherally-selective anti-androgen: preclinical studies". Hormone Research. 32 (Suppl 1): 69–76. doi:10.1159/000181315 (inactive 11 November 2024). PMID 2533159.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Furr BJ, Valcaccia B, Curry B, Woodburn JR, Chesterson G, Tucker H (June 1987). "ICI 176,334: a novel non-steroidal, peripherally selective antiandrogen". The Journal of Endocrinology. 113 (3): R7-9. doi:10.1677/joe.0.113R007. PMID 3625091.
- ^ Soloway MS, Schellhammer PF, Smith JA, Chodak GW, Vogelzang NJ, Kennealey GT (December 1995). "Bicalutamide in the treatment of advanced prostatic carcinoma: a phase II noncomparative multicenter trial evaluating safety, efficacy and long-term endocrine effects of monotherapy". The Journal of Urology. 154 (6): 2110–4. doi:10.1016/S0022-5347(01)66709-0. PMID 7500470.
- ^ Gao W, Dalton JT (March 2007). "Expanding the therapeutic use of androgens via selective androgen receptor modulators (SARMs)". Drug Discovery Today. 12 (5–6): 241–8. doi:10.1016/j.drudis.2007.01.003. PMC 2072879. PMID 17331889.
- ^ Mason M (August 2006). "What implications do the tolerability profiles of antiandrogens and other commonly used prostate cancer treatments have on patient care?". Journal of Cancer Research and Clinical Oncology. 132 (Suppl 1): S27-35. doi:10.1007/s00432-006-0134-4. PMID 16896883. S2CID 19685819.
- ^ Veldhuis JD, Takahashi PY, Keenan DM, Liu PY, Mielke KL, Weist SM (October 2010). "Age disrupts androgen receptor-modulated negative feedback in the gonadal axis in healthy men". Am J Physiol Endocrinol Metab. 299 (4): E675–82. doi:10.1152/ajpendo.00300.2010. PMC 2957871. PMID 20682842.
- ^ a b c d Lemke TL, Williams DA (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1372–1373. ISBN 978-1-60913-345-0. Archived from the original on 3 May 2016.
- ^ Butler SK, Govindan R (25 October 2010). Essential Cancer Pharmacology: The Prescriber's Guide. Lippincott Williams & Wilkins. pp. 49–. ISBN 978-1-60913-704-5. Archived from the original on 14 January 2023. Retrieved 27 September 2016.
- ^ Fradet Y (February 2004). "Bicalutamide (Casodex) in the treatment of prostate cancer". Expert Review of Anticancer Therapy. 4 (1): 37–48. doi:10.1586/14737140.4.1.37. PMID 14748655. S2CID 34153031.
In contrast, the incidence of diarrhea was comparable between the bicalutamide and placebo groups (6.3 vs. 6.4%, respectively) in the EPC program [71].
- ^ Sharma K, Pawar GV, Giri S, Rajagopal S, Mullangi R (2012). "Development and validation of a highly sensitive LC-MS/MS-ESI method for the determination of bicalutamide in mouse plasma: application to a pharmacokinetic study". Biomedical Chromatography. 26 (12): 1589–95. doi:10.1002/bmc.2736. PMID 22495777.
- ^ Anderson PO, Knoben JE, Troutman WG (22 August 2001). Handbook of Clinical Drug Data. Canadian Medical Association Journal. Vol. 128. McGraw Hill Professional. p. 245. ISBN 978-0-07-138942-6. PMC 1875767. PMID 20313924.
With an oral dose of 50 mg/day, bicalutamide attains a peak serum level of 8.9 mg/L (21 μmol/L) 31 hr after a dose at steady state. CI of (R)-bicalutamide is 0.32 L/hr. The active (R)-enantiomer of bicalutamide is oxidized to an inactive metabolite, which, like the inactive (S)-enantiomer, is glucuronidated and cleared rapidly by elimination in the urine and feces.165
- ^ a b c d McPherson EM (22 October 2013). Pharmaceutical Manufacturing Encyclopedia (3rd ed.). William Andrew Publishing. pp. 627, 1695. ISBN 978-0-8155-1856-3. Archived from the original on 9 June 2016.
- ^ Komsta L, Waksmundzka-Hajnos M, Sherma J (20 December 2013). Thin Layer Chromatography in Drug Analysis. CRC Press. pp. 652–. ISBN 978-1-4665-0715-9.
- ^ Sancheti PP, Vyas VM, Shah M, Karekar P, Pore YV (2008). "Spectrophotometric estimation of bicalutamide in tablets". Indian Journal of Pharmaceutical Sciences. 70 (6): 810–2. doi:10.4103/0250-474X.49131. PMC 3040883. PMID 21369450.
- ^ a b c d e Mohler ML, Bohl CE, Jones A, Coss CC, Narayanan R, He Y, Hwang DJ, Dalton JT, Miller DD (June 2009). "Nonsteroidal selective androgen receptor modulators (SARMs): dissociating the anabolic and androgenic activities of the androgen receptor for therapeutic benefit". Journal of Medicinal Chemistry. 52 (12): 3597–617. doi:10.1021/jm900280m. PMID 19432422.
[C]linically relevant antiandrogens currently are nonsteroidal anilide derivatives. Antiandrogens used for prostate cancer include the monoarylpropionamide flutamide (1) (a prodrug of hydroxyflutamide (2)),29–31 the hydantoin nilutamide(3),32–34 and the diarylpropionamide bicalutamide (4) (Chart1).35–37
- ^ a b Bégué J, Bonnet-Delpon D (2 June 2008). Bioorganic and Medicinal Chemistry of Fluorine. John Wiley & Sons. pp. 327–. ISBN 978-0-470-28187-1. Archived from the original on 12 January 2023. Retrieved 27 September 2016.
- ^ Ball AL, Kamalian L, Alfirevic A, Lyon JJ, Chadwick AE (July 2016). "Identification of the Additional Mitochondrial Liabilities of 2-Hydroxyflutamide When Compared With its Parent Compound, Flutamide in HepG2 Cells". Toxicological Sciences. 153 (2): 341–351. doi:10.1093/toxsci/kfw126. PMC 5036617. PMID 27413113.
- ^ a b Hermkens PH, Kamp S, Lusher S, Veeneman GH (July 2006). "Non-steroidal steroid receptor modulators". IDrugs. 9 (7): 488–94. doi:10.2174/0929867053764671. PMID 16821162.
- ^ Avram MR, Rogers NE (30 November 2009). Hair Transplantation. Cambridge University Press. pp. 11–. ISBN 978-1-139-48339-1. Archived from the original on 14 January 2023. Retrieved 3 November 2016.
- ^ Haber RS, Stough DB (2006). Hair Transplantation. Elsevier Health Sciences. pp. 6–7. ISBN 978-1-4160-3104-8. Archived from the original on 4 July 2014. Retrieved 28 May 2012.
- ^ Kawahara T, Minamoto H (2014). "Androgen Receptor Antagonists in the Treatment of Prostate Cancer". Clinical Immunology, Endocrine & Metabolic Drugs. 1 (1): 11–19. doi:10.2174/22127070114019990002.
- ^ Moilanen AM, Riikonen R, Oksala R, Ravanti L, Aho E, Wohlfahrt G, Nykänen PS, Törmäkangas OP, Palvimo JJ, Kallio PJ (2015). "Discovery of ODM-201, a new-generation androgen receptor inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies". Sci Rep. 5: 12007. Bibcode:2015NatSR...512007M. doi:10.1038/srep12007. PMC 4490394. PMID 26137992.
- ^ a b c Segal S, Narayanan R, Dalton JT (April 2006). "Therapeutic potential of the SARMs: revisiting the androgen receptor for drug discovery". Expert Opinion on Investigational Drugs. 15 (4): 377–87. doi:10.1517/13543784.15.4.377. PMID 16548787. S2CID 31787187.
Structural modifications of bicalutamide led to the discovery of the first nonsteroidal androgens (the aryl propionamides) in 1998. Lead compounds in this class (denoted S1 and S4 in published literature) not only bind to the AR with high affinity (low nanomolar range), but also demonstrate tissue selectivity in animal models [46,50].
- ^ Yin D, Gao W, Kearbey JD, Xu H, Chung K, He Y, Marhefka CA, Veverka KA, Miller DD, Dalton JT (March 2003). "Pharmacodynamics of selective androgen receptor modulators". The Journal of Pharmacology and Experimental Therapeutics. 304 (3): 1334–40. doi:10.1124/jpet.102.040840. PMC 2040238. PMID 12604714.
- ^ Ottow E, Weinmann H (8 September 2008). Nuclear Receptors as Drug Targets. John Wiley & Sons. pp. 257–258. ISBN 978-3-527-62330-3. Archived from the original on 14 January 2023. Retrieved 1 December 2016.
- ^ a b c d Parent EE, Dence CS, Jenks C, Sharp TL, Welch MJ, Katzenellenbogen JA (2007). "Synthesis and biological evaluation of [18F]bicalutamide, 4-[76Br]bromobicalutamide, and 4-[76Br]bromo-thiobicalutamide as non-steroidal androgens for prostate cancer imaging". J. Med. Chem. 50 (5): 1028–40. doi:10.1021/jm060847r. PMID 17328524.
- ^ a b c Dierckx RA, Otte A, de Vries EF, van Waarde A, Luiten PG (15 February 2014). PET and SPECT of Neurobiological Systems. Springer Science & Business Media. pp. 394–. ISBN 978-3-642-42014-6.
- ^ a b de Jesus Cortez F, Nguyen P, Truillet C, Tian B, Kuchenbecker KM, Evans MJ, Webb P, Jacobson MP, Fletterick RJ, England PM (2017). "Development of 5N-Bicalutamide, a High-Affinity Reversible Covalent Antiandrogen". ACS Chem. Biol. 12 (12): 2934–2939. doi:10.1021/acschembio.7b00702. PMID 28981251. S2CID 24974359.
- ^ US Patent 10053433B2, England, Pamela M.; Fletterick, R. J. & Kuchenbecker, K. et al., published 2016
- ^ a b c Tucker H, Crook JW, Chesterson GJ (1988). "Nonsteroidal antiandrogens. Synthesis and structure-activity relationships of 3-substituted derivatives of 2-hydroxypropionanilides". Journal of Medicinal Chemistry. 31 (5): 954–9. doi:10.1021/jm00400a011. PMID 3361581.
- ^ James KD, Ekwuribe NN (2002). "A Two-step Synthesis of the Anti-cancer Drug (R,S)-Bicalutamide". Synthesis. 2002 (7): 850–2. doi:10.1055/s-2002-28508.
- ^ US application 2006/0041161, Pizzetti E, Vigano E, Lussana M, Landonio E, "Procedure for the synthesis of bicalutamide", published 23 February 2006 Archived 4 January 2018 at the Wayback Machine
- ^ Chand M, Shukla AK (2012). Novel Synthesis of Bicalutamide Drug Substance and their Impurities using Imidazolium Type of Ionic Liquid (Report). doi:10.2139/ssrn.2160199. SSRN 2160199.
- ^ Diamanti-Kandarakis E (September 1999). "Current aspects of antiandrogen therapy in women". Current Pharmaceutical Design. 5 (9): 707–23. doi:10.2174/1381612805666230111201150. PMID 10495361. Archived from the original on 10 January 2023. Retrieved 27 September 2016.
Several trials demonstrated complete clearing of acne with flutamide [62,77]. Flutamide used in combination with an [oral contraceptive], at a dose of 500mg/d, flutamide caused a dramatic decrease (80%) in total acne, seborrhea and hair loss score after only 3 months of therapy [53]. When used as a monotherapy in lean and obese PCOS, it significantly improves the signs of hyperandrogenism, hirsutism and particularly acne [48]. [...] flutamide 500mg/d combined with an [oral contraceptive] caused an increase in cosmetically acceptable hair density, in sex of seven women suffering from diffuse androgenetic alopecia [53].
- ^ Denis LJ, Griffiths K, Kaisary AV, Murphy GP (1 March 1999). Textbook of Prostate Cancer: Pathology, Diagnosis and Treatment: Pathology, Diagnosis and Treatment. CRC Press. pp. 55, 279–280. ISBN 978-1-85317-422-3. Archived from the original on 3 June 2016.
- ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 573–. ISBN 978-1-4757-2085-3. Archived from the original on 15 May 2016.
- ^ Furr BJ (1998). "Research on reproductive medicine in the pharmaceutical industry". Hum Fertil (Camb). 1 (1): 56–63. doi:10.1080/1464727982000198131. PMID 11844311.
- ^ Cadilla R, Turnbull P (2006). "Selective androgen receptor modulators in drug discovery: medicinal chemistry and therapeutic potential". Curr Top Med Chem. 6 (3): 245–70. doi:10.2174/156802606776173456. PMID 16515480. Archived from the original on 18 August 2020. Retrieved 11 December 2019.
- ^ Engel J, Kleemann A, Kutscher B, Reichert D (2009). Pharmaceutical Substances: Syntheses, Patents and Applications of the most relevant APIs (5th ed.). Thieme. pp. 153–154. ISBN 978-3-13-179275-4.
- ^ Furr BJ, Valcaccia B, Curry B, Woodburn JR, Chesterson G, Tucker H (June 1987). "ICI 176,334: a novel non-steroidal, peripherally selective antiandrogen". The Journal of Endocrinology. 113 (3): R7-9. doi:10.1677/joe.0.113r007. PMID 3625091.
- ^ Newling DW (1990). "The response of advanced prostatic cancer to a new non-steroidal antiandrogen: results of a multicenter open phase II study of Casodex. European/Australian Co-operative Group". European Urology. 18 (Suppl 3): 18–21. doi:10.1159/000463973. PMID 2094607.
- ^ The United States Patents Quarterly. Associated Industry Publications. 1997. Archived from the original on 14 January 2023. Retrieved 27 September 2016.
- ^ Chaurasiya A, Singh AK, Upadhyay SC, Asati D, Ahmad FJ, Mukherjee R, Khar (2012). "Lipidic Nanocarrier for Oral Bioavailability Enhancement of an Anticancer Agent: Formulation Design and Evaluation". Advanced Science Letters. 11 (1): 43–52. doi:10.1166/asl.2012.2170. ISSN 1936-6612.
- ^ Klotz L (May 2006). "Combined androgen blockade: an update". The Urologic Clinics of North America. 33 (2): 161–6, v–vi. doi:10.1016/j.ucl.2005.12.001. PMID 16631454.
- ^ Gohil K (August 2015). "Exciting Therapies Ahead in Prostate Cancer". P & T. 40 (8): 530–531. PMC 4517537. PMID 26236143.
- ^ Kolvenbag GJ, Iversen P, Newling DW (2001). "Antiandrogen monotherapy: a new form of treatment for patients with prostate cancer". Urology. 58 (2 Suppl 1): 16–23. doi:10.1016/s0090-4295(01)01237-7. PMID 11502439.
- ^ Carswell CI, Figgitt DP (2002). "Bicalutamide: in early-stage prostate cancer". Drugs. 62 (17): 2471–79, discussion 2480–1. doi:10.2165/00003495-200262170-00006. PMID 12421104. S2CID 195690919.
- ^ Jasmin C, Capanna R, Coia L, Coleman R, Saillant G (27 September 2005). Textbook of Bone Metastases. John Wiley & Sons. pp. 493–. ISBN 978-0-470-01160-7. Archived from the original on 14 January 2023. Retrieved 27 September 2016.
- ^ United Nations (2005). Consolidated List of Products Whose Consumption And/or Sale Have Been Banned, Withdrawn, Severely Restricted Or Not Approved by Governments: Pharmaceuticals. United Nations Publications. pp. 4–. ISBN 978-92-1-130241-7.
- ^ Bono AV (2004). "Overview of Current Treatment Strategies in Prostate Cancer". European Urology Supplements. 3 (1): 2–7. doi:10.1016/j.eursup.2003.12.002.
The Canadian Health Authorities have withdrawn the approval for antiandrogen monotherapy with bicalutamide for the treatment of localised prostate cancer [5]. Several European countries have also withdrawn approval for bicalutamide for this indication.
- ^ a b Moul JW (August 2009). "Twenty years of controversy surrounding combined androgen blockade for advanced prostate cancer". Cancer. 115 (15): 3376–8. doi:10.1002/cncr.24393. PMID 19484788. S2CID 5670663.
- ^ Kampel LJ (20 March 2012). Dx/Rx: Prostate Cancer. Jones & Bartlett Publishers. pp. 178–. ISBN 978-0-7637-9453-8. Archived from the original on 14 January 2023. Retrieved 27 September 2016.
- ^ Tobias JS, Hochhauser D (3 October 2014). Cancer and its Management. Wiley. pp. 379–. ISBN 978-1-118-46871-5.
- ^ Neal DE (1994). Tumours in Urology. Springer Science & Business Media. pp. 233–. ISBN 978-1-4471-2086-5. Archived from the original on 27 April 2016.
- ^ Regitz-Zagrosek V (2 October 2012). Sex and Gender Differences in Pharmacology. Springer Science & Business Media. pp. 575–. ISBN 978-3-642-30725-6. Archived from the original on 24 June 2016.
- ^ Bohl CE, Gao W, Miller DD, Bell CE, Dalton JT (April 2005). "Structural basis for antagonism and resistance of bicalutamide in prostate cancer". Proceedings of the National Academy of Sciences of the United States of America. 102 (17): 6201–6. Bibcode:2005PNAS..102.6201B. doi:10.1073/pnas.0500381102. PMC 1087923. PMID 15833816.
- ^ Kolinsky M, de Bono JS (2016). "The Ongoing Challenges of Targeting the Androgen Receptor". European Urology. 69 (5): 841–3. doi:10.1016/j.eururo.2015.10.052. PMID 26585581. Archived from the original on 20 September 2016. Retrieved 9 September 2016.
- ^ "Bicalutamide". Kyoto Encyclopedia of Genes and Genomes (KEGG). Archived from the original on 26 November 2016.
- ^ a b Morton IK, Hal JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 51–. ISBN 978-94-011-4439-1. Archived from the original on 14 May 2016.
- ^ Ganellin CR, Triggle DJ (21 November 1996). Dictionary of Pharmacological Agents. CRC Press. pp. 570–. ISBN 978-0-412-46630-4. Archived from the original on 7 May 2016.
- ^ The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances (PDF) (Report). World Health Organisation. 2013. Archived from the original (PDF) on 9 August 2017. Retrieved 6 June 2020.
- ^ "21 results for Name/Synonym ends with LUTAMIDE". Drug Information Portal. Archived from the original on 27 July 2020. Retrieved 19 November 2017.
- ^ Ferraro MB, Orendt AM, Facelli JC (19 September 2009). "Parallel Genetic Algorithms for Crystal Structure Prediction: Successes and Failures in Predicting Bicalutamide Polymorphs". In Huang D, Jo K, Lee H, Kang H, Bevilacqua V (eds.). Emerging Intelligent Computing Technology and Applications: 5th International Conference on Intelligent Computing, ICIC 2009 Ulsan, South Korea, September 16–19, 2009 Proceedings. Lecture Notes in Computer Science. Vol. 5754. Springer. pp. 120–. doi:10.1007/978-3-642-04070-2_14. ISBN 978-3-642-04070-2. Archived from the original on 14 January 2023. Retrieved 27 September 2016.
- ^ Dhas NL, Ige PP, Kudarha RR (2015). "Design, optimization and in-vitro study of folic acid conjugated-chitosan functionalized PLGA nanoparticle for delivery of bicalutamide in prostate cancer". Powder Technology. 283: 234–245. doi:10.1016/j.powtec.2015.04.053.
- ^ Ramadan WH, Kabbara WK, Al Basiouni Al Masri HS (2015). "Enzalutamide for patients with metastatic castration-resistant prostate cancer". OncoTargets and Therapy. 8: 871–6. doi:10.2147/OTT.S80488. PMC 4407758. PMID 25945058.
- ^ a b Stuhan MA (2 April 2013). Understanding Pharmacology for Pharmacy Technicians. ASHP. pp. 268–. ISBN 978-1-58528-360-6. Archived from the original on 14 January 2023. Retrieved 5 November 2016.
- ^ a b Allan GF, Sui Z (2003). "Therapeutic androgen receptor ligands". Nucl Recept Signal. 1: e009. doi:10.1621/nrs.01009. PMC 1402218. PMID 16604181.
- ^ Emans SJ, Laufer MR (5 January 2012). Emans, Laufer, Goldstein's Pediatric and Adolescent Gynecology. Lippincott Williams & Wilkins. pp. 365–. ISBN 978-1-4511-5406-1. Archived from the original on 16 May 2016.
Therapy with GnRH analogs is expensive and requires intramuscular injections of depot formulations, the insert of a subcutaneous implant yearly, or, much less commonly, daily subcutaneous injections.
- ^ Hillard PJ (29 March 2013). Practical Pediatric and Adolescent Gynecology. John Wiley & Sons. pp. 182–. ISBN 978-1-118-53857-9. Archived from the original on 14 January 2023. Retrieved 27 September 2016.
Treatment is expensive, with costs typicall in the range of $10,000–$15,000 per year.
- ^ a b c "Annual Report and Form 20-F 2007" (PDF). AstraZeneca.
- ^ a b "Actavis Generic Prostate Cancer Drug Bicalutamide First to Market in UK, Germany, France". Press Release. AstraZeneca, Actavis. 10 July 2008. Archived from the original on 28 August 2021. Retrieved 1 July 2017.
- ^ a b "Hormonal Therapies" (PDF). Future Oncology. 2 (2–3): 306. June 1996. Archived from the original (PDF) on 9 September 2017. Retrieved 1 July 2017.
- ^ a b "Zeneca of Britain Posts Strong Drug Profits". The New York Times. 12 March 1997.
- ^ a b "Annual Report and Form 20-F 2001" (PDF). AstraZeneca.
- ^ a b "Annual Report and Form 20-F 2004" (PDF). AstraZeneca.
- ^ a b "Annual Report and Form 20-F 2010" (PDF). AstraZeneca.
- ^ a b "Annual Report and Form 20-F 2013" (PDF). AstraZeneca.
- ^ a b "Annual Report and Form 20-F 2016" (PDF). AstraZeneca. Archived (PDF) from the original on 3 April 2017.
- ^ a b "AstraZeneca Full-Year 2017 Results" (PDF). AstraZeneca.
- ^ a b "AstraZeneca Full-Year 2018 Results" (PDF). AstraZeneca.
- ^ Yagiela JA, Dowd FJ, Johnson B, Mariotti A, Neidle EA (19 March 2010). Pharmacology and Therapeutics for Dentistry. Elsevier Health Sciences. pp. 851–. ISBN 978-0-323-07824-5.
- ^ Hepler CD, Segal R (25 February 2003). Preventing Medication Errors and Improving Drug Therapy Outcomes: A Management Systems Approach. CRC Press. pp. 136–137. ISBN 978-0-203-01073-0.
- ^ Dukes G, Dukes MN, Mildred M, Swartz B (January 1998). Responsibility for Drug-induced Injury: A Reference Book for Lawyers, the Health Professions and Manufacturers. IOS Press. pp. 241–8. ISBN 978-90-5199-387-5.
- ^ Wang LG, Mencher SK, McCarron JP, Ferrari AC (2004). "The biological basis for the use of an anti-androgen and a 5-alpha-reductase inhibitor in the treatment of recurrent prostate cancer: Case report and review". Oncology Reports. 11 (6): 1325–9. doi:10.3892/or.11.6.1325. PMID 15138573.
- ^ Tay MH, Kaufman DS, Regan MM, Leibowitz SB, George DJ, Febbo PG, Manola J, Smith MR, Kaplan ID, Kantoff PW, Oh WK (2004). "Finasteride and bicalutamide as primary hormonal therapy in patients with advanced adenocarcinoma of the prostate". Annals of Oncology. 15 (6): 974–8. doi:10.1093/annonc/mdh221. PMID 15151957.
- ^ Merrick GS, Butler WM, Wallner KE, Galbreath RW, Allen ZA, Kurko B (2006). "Efficacy of neoadjuvant bicalutamide and dutasteride as a cytoreductive regimen before prostate brachytherapy". Urology. 68 (1): 116–20. doi:10.1016/j.urology.2006.01.061. PMID 16844453.
- ^ Sartor O, Gomella LG, Gagnier P, Melich K, Dann R (2009). "Dutasteride and bicalutamide in patients with hormone-refractory prostate cancer: the Therapy Assessed by Rising PSA (TARP) study rationale and design". The Canadian Journal of Urology. 16 (5): 4806–12. PMID 19796455.
- ^ Chu FM, Sartor O, Gomella L, Rudo T, Somerville MC, Hereghty B, Manyak MJ (2015). "A randomised, double-blind study comparing the addition of bicalutamide with or without dutasteride to GnRH analogue therapy in men with non-metastatic castrate-resistant prostate cancer". European Journal of Cancer. 51 (12): 1555–69. doi:10.1016/j.ejca.2015.04.028. PMID 26048455.
- ^ Gaudet M, Vigneault É, Foster W, Meyer F, Martin AG (2016). "Randomized non-inferiority trial of Bicalutamide and Dutasteride versus LHRH agonists for prostate volume reduction prior to I-125 permanent implant brachytherapy for prostate cancer". Radiotherapy and Oncology. 118 (1): 141–7. doi:10.1016/j.radonc.2015.11.022. PMID 26702991.
- ^ Dijkstra S, Witjes WP, Roos EP, Vijverberg PL, Geboers AD, Bruins JL, Smits GA, Vergunst H, Mulders PF (2016). "The AVOCAT study: Bicalutamide monotherapy versus combined bicalutamide plus dutasteride therapy for patients with locally advanced or metastatic carcinoma of the prostate-a long-term follow-up comparison and quality of life analysis". SpringerPlus. 5: 653. doi:10.1186/s40064-016-2280-8. PMC 4870485. PMID 27330919.
- ^ Fujimura T, Takayama K, Takahashi S, Inoue S (January 2018). "Estrogen and Androgen Blockade for Advanced Prostate Cancer in the Era of Precision Medicine". Cancers. 10 (2): 29. doi:10.3390/cancers10020029. PMC 5836061. PMID 29360794.
- ^ Ho TH, Nunez-Nateras R, Hou YX, Bryce AH, Northfelt DW, Dueck AC, et al. (April 2017). "A Study of Combination Bicalutamide and Raloxifene for Patients With Castration-Resistant Prostate Cancer". Clinical Genitourinary Cancer. 15 (2): 196–202.e1. doi:10.1016/j.clgc.2016.08.026. PMID 27771244. S2CID 19043552.
- ^ Translational Breast Cancer Research Consortium (TBCRC) (2012). "Targeting the androgen receptor (AR) in women with AR+ ER-/PR- metastatic breast cancer (MBC)". J Clin Oncol (suppl): abstract 1006. Archived from the original on 10 July 2015.
- ^ Clinical trial number NCT00468715 for "Bicalutamide in Treating Patients With Metastatic Breast Cancer" at ClinicalTrials.gov
- ^ Gucalp A, Tolaney S, Isakoff SJ, Ingle JN, Liu MC, Carey LA, Blackwell K, Rugo H, Nabell L, Forero A, Stearns V, Doane AS, Danso M, Moynahan ME, Momen LF, Gonzalez JM, Akhtar A, Giri DD, Patil S, Feigin KN, Hudis CA, Traina TA, et al. (Translational Breast Cancer Research Consortium (TBCRC 011)) (October 2013). "Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer". Clinical Cancer Research. 19 (19): 5505–12. doi:10.1158/1078-0432.CCR-12-3327. PMC 4086643. PMID 23965901.
- ^ Caiazza F, Murray A, Madden SF, Synnott NC, Ryan EJ, O'Donovan N, Crown J, Duffy MJ (April 2016). "Preclinical evaluation of the AR inhibitor enzalutamide in triple-negative breast cancer cells". Endocrine-Related Cancer. 23 (4): 323–34. doi:10.1530/ERC-16-0068. PMID 26932782.
- ^ Traina TA, et al. (2015). "Results from a phase 2 study of enzalutamide (ENZA), an androgen receptor (AR) inhibitor, in advanced AR+ triple-negative breast cancer (TNBC)". Journal of Clinical Oncology. 33 (suppl): abstr 1003. doi:10.1200/jco.2015.33.15_suppl.1003. Archived from the original on 30 May 2016.
- ^ Levine D, Park K, Juretzka M, Esch J, Hensley M, Aghajanian C, Lewin S, Konner J, Derosa F, Spriggs D, Iasonos A, Sabbatini P (December 2007). "A phase II evaluation of goserelin and bicalutamide in patients with ovarian cancer in second or higher complete clinical disease remission". Cancer. 110 (11): 2448–56. doi:10.1002/cncr.23072. PMID 17918264. S2CID 21161915.
- ^ a b c Becker KL (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. p. 1209. ISBN 978-0-7817-1750-2. Archived from the original on 28 June 2014.
- ^ a b c d Lepor H (1993). "Medical therapy for benign prostatic hyperplasia". Urology. 42 (5): 483–501. doi:10.1016/0090-4295(93)90258-c. PMID 7694413.
The clinically significant adverse events reported in the casodex group included breast tenderness (93%), breast enlargement (54%), and sexual dysfunction (60%). In none of the patients in the placebo group did any of these adverse events develop. None of the subjects discontinued therapy owing to an adverse event.
- ^ Lee M, Sharifi R (1997). "Benign prostatic hyperplasia: diagnosis and treatment guideline". Ann Pharmacother. 31 (4): 481–6. doi:10.1177/106002809703100415. PMID 9101011. S2CID 20498549.
- ^ Kenny B, Ballard S, Blagg J, Fox D (1997). "Pharmacological options in the treatment of benign prostatic hyperplasia". J. Med. Chem. 40 (9): 1293–315. doi:10.1021/jm960697s. PMID 9135028.
- ^ McCoy J, Wambier CG, Vano-Galvan S, Shapiro J, Sinclair R, Müller Ramos P, Washenik K, Andrade M, Herrera S, Goren A (April 2020). "Racial Variations in COVID-19 Deaths May Be Due to Androgen Receptor Genetic Variants Associated with Prostate Cancer and Androgenetic Alopecia. Are Anti-Androgens a Potential Treatment for COVID-19?". J Cosmet Dermatol. 19 (7): 1542–1543. doi:10.1111/jocd.13455. PMC 7267367. PMID 32333494.
- ^ "A Phase II Trial to Promote Recovery from COVID-19 with Endocrine Therapy". 2 March 2021.
- ^ Bonagura JD, Twedt DC (1 December 2013). Kirk's Current Veterinary Therapy XV. Elsevier Health Sciences. p. 908. ISBN 978-0-323-22762-9.
- ^ Mitchell MA, Tully TN (2009). Manual of Exotic Pet Practice. Elsevier Health Sciences. p. 363. ISBN 978-1-4160-0119-5.
- ^ a b Pilny AA (9 February 2014). Endocrinology, An Issue of Veterinary Clinics: Exotic Animal Practice. Elsevier Health Sciences. pp. 16–17. ISBN 978-0-323-26419-8.
In ferrets, 5 mg/kg [of bicalutamide] orally every 24 hours has been used clinically, but no controlled toxicologic or pharmacologic studies have been published at this time.
- ^ Fox JG, Marini RP (26 March 2014). Biology and Diseases of the Ferret. Wiley. p. 980. ISBN 978-1-118-78273-6.
Other agents have been proposed for medical management of [adrenal-associated endocrinopathy] but have not been studied. Possibly medications include the androgen receptor blockers flutamide and bicalutamide, the anti-androgen finasteride, estrogen-inhibiting anastrozole, and another GnRH analog, goserelin. [...] None of these drugs have been tested in controlled clinical trials in ferrets.
Further reading
[edit]- "Bicalutamide". LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. 2017. PMID 31643303. NBK547970 – via NCBI Bookshelf.
- Blackledge GR (1996). "Clinical progress with a new antiandrogen, Casodex (bicalutamide)". European Urology. 29 (Suppl 2): 96–104. doi:10.1159/000473847. PMID 8717470.
- Cockshott ID (2004). "Bicalutamide: clinical pharmacokinetics and metabolism". Clinical Pharmacokinetics. 43 (13): 855–78. doi:10.2165/00003088-200443130-00003. PMID 15509184. S2CID 29912565.
- Fradet Y (February 2004). "Bicalutamide (Casodex) in the treatment of prostate cancer". Expert Review of Anticancer Therapy. 4 (1): 37–48. doi:10.1586/14737140.4.1.37. PMID 14748655. S2CID 34153031.
- Furr BJ (June 1995). "Casodex: preclinical studies and controversies". Annals of the New York Academy of Sciences. 761 (1): 79–96. Bibcode:1995NYASA.761...79F. doi:10.1111/j.1749-6632.1995.tb31371.x. PMID 7625752. S2CID 37242269.
- Furr BJ, Tucker H (January 1996). "The preclinical development of bicalutamide: pharmacodynamics and mechanism of action". Urology. 47 (1A Suppl): 13–25, discussion 29–32. doi:10.1016/S0090-4295(96)80003-3. PMID 8560673.
- Kolvenbag GJ, Blackledge GR (January 1996). "Worldwide activity and safety of bicalutamide: a summary review". Urology. 47 (1A Suppl): 70–9, discussion 80–4. doi:10.1016/s0090-4295(96)80012-4. PMID 8560681.
- Schellhammer PF, Davis JW (March 2004). "An evaluation of bicalutamide in the treatment of prostate cancer". Clinical Prostate Cancer. 2 (4): 213–9. doi:10.3816/CGC.2004.n.002. PMID 15072604.
- Tucker H, Crook JW, Chesterson GJ (1988). "Nonsteroidal antiandrogens. Synthesis and structure-activity relationships of 3-substituted derivatives of 2-hydroxypropionanilides". J. Med. Chem. 31 (5): 954–9. doi:10.1021/jm00400a011. PMID 3361581.
- Wellington K, Keam SJ (2006). "Bicalutamide 150mg: a review of its use in the treatment of locally advanced prostate cancer". Drugs. 66 (6): 837–50. doi:10.2165/00003495-200666060-00007. PMID 16706554. S2CID 46966712.
- 27-Hydroxylase inhibitors
- Anilides
- Anti-acne preparations
- Antiprogestogens
- Drugs developed by AstraZeneca
- Benzonitriles
- Benzosulfones
- Bicalutamide
- CYP3A4 inhibitors
- Hair loss medications
- Hair removal
- Hepatotoxins
- Hormonal antineoplastic drugs
- Nonsteroidal antiandrogens
- Progonadotropins
- Prostate cancer
- Tertiary alcohols
- Trifluoromethyl compounds
- Veterinary drugs
- World Health Organization essential medicines
- 4-Fluorophenyl compounds