TMEM70
| TMEM70 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | TMEM70, MC5DN2, transmembrane protein 70 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 612418; MGI: 1915068; HomoloGene: 9890; GeneCards: TMEM70; OMA:TMEM70 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Transmembrane protein 70 is a protein that in humans is encoded by the TMEM70 gene. It is a transmembrane protein located in the mitochondrial inner membrane involved in the assembly of the F1 and Fo structural subunits of ATP synthase.[5] Mutations in this gene have been associated with neonatal mitochondrial encephalo-cardiomyopathy due to ATP synthase (Complex V) deficiency, causing a wide variety of symptoms including 3-methylglutaconic aciduria, lactic acidosis, mitochondrial myopathy, and cardiomyopathy.[6][7]
Structure
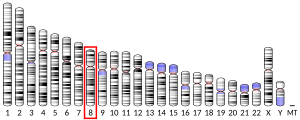
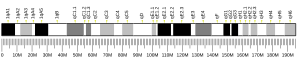
[edit]The TMEM70 gene has 4 exons and is located on the q arm of chromosome 8 in position 21.11 and spans 6,642 base pairs.[5] The gene produces a 29 kDa protein composed of 260 amino acids.[8][9] The encoded protein is a multi-pass transmembrane protein localized to the mitochondrial inner membrane.[10][11] It contains two putative transmembrane regions and the conserved domain DUF1301.[12][6]
Function
[edit]The encoded protein is involved in the assembly of the F1 and Fo structural subunits of ATP synthase.[5]
Clinical significance
[edit]Mutations in the TMEM70 gene have been associated with neonatal mitochondrial encephalocardiomyopathy due to nuclear type 2 Complex V (ATP synthase) deficiency.[5] There are a wide variety of possible symptoms depending on the mutation, including 3-methylglutaconic aciduria, dysmorphic features, psychomotor retardation, hypotonia, growth retardation, mitochondrial myopathy and cardiomyopathy, hepatomegaly, hypoplastic kidneys, and elevated lactate levels in urine, plasma, and cerebrospinal fluid.[11][7]
Most notably, a c.317-2A→G mutation in the splice site of intron 2 of this gene caused aberrant splicing and the loss of the TMEM70 transcript.[13] This resulted in ATP synthase deficiency symptomized by apneoic spells, hypertrophic cardiomyopathy, profound lactic acidosis, hyperammonaemia, psychomotor retardation, 3-methylglutaconic aciduria, failure to thrive, and severe muscular hypotonia. Also noted in some patients were hypospadias, intrauterine growth retardation, microcephaly and cryptorchidism, but most patients did not survive the neonatal period.[14]
Another mutation (c.366A>T) in the second exon of this gene caused an amino acid substitution (Y112X), resulting in Nuclear Type 2 Mitochondrial Complex V deficiency symptomized by lactic acidosis, psychomotor retardation, facial dysmorphism, hypertrophic cardiomyopathy, and hypospadia.[15][6]
A study of mitochondrial morphology in patients with mutations in this gene revealed disorganization of the mitochondrial nucleoid. Mitochondria were abnormal, with whorled cristae and disrupted nucleoid clusters of mtDNA. The nucleoids and mitochondrial respiratory chain complexes were confined to the outer rings of the whorls.[7]
Interactions
[edit]The encoded protein has protein-protein interactions with RAB2A, PHC2, NDUFAF8, NDUFS5, COX6B1, ECSIT, NDUFAF4, and COA3.[16]
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000175606 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000025940 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b c d "Entrez Gene: Transmembrane protein 70". Retrieved 2018-08-14.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b c Online Mendelian Inheritance in Man (OMIM): TMEM70 - 612418
- ^ a b c Cameron JM, Levandovskiy V, Mackay N, Ackerley C, Chitayat D, Raiman J, et al. (January 2011). "Complex V TMEM70 deficiency results in mitochondrial nucleoid disorganization". Mitochondrion. 11 (1): 191–9. doi:10.1016/j.mito.2010.09.008. PMID 20920610.
- ^ Zong NC, Li H, Li H, Lam MP, Jimenez RC, Kim CS, et al. (October 2013). "Integration of cardiac proteome biology and medicine by a specialized knowledgebase". Circulation Research. 113 (9): 1043–53. doi:10.1161/CIRCRESAHA.113.301151. PMC 4076475. PMID 23965338.
- ^ "TMEM70 - Transmembrane protein 70, mitochondrial". Cardiac Organellar Protein Atlas Knowledgebase (COPaKB).[permanent dead link]
- ^ "TMEM70 - Transmembrane protein 70, mitochondrial precursor - Homo sapiens (Human) - TMEM70 gene & protein". www.uniprot.org. Retrieved 2018-08-15.
 This article incorporates text available under the CC BY 4.0 license.
This article incorporates text available under the CC BY 4.0 license.
- ^ a b "UniProt: the universal protein knowledgebase". Nucleic Acids Research. 45 (D1): D158–D169. January 2017. doi:10.1093/nar/gkw1099. PMC 5210571. PMID 27899622.
- ^ Cízková A, Stránecký V, Mayr JA, Tesarová M, Havlícková V, Paul J, et al. (November 2008). "TMEM70 mutations cause isolated ATP synthase deficiency and neonatal mitochondrial encephalocardiomyopathy". Nature Genetics. 40 (11): 1288–90. doi:10.1038/ng.246. PMID 18953340. S2CID 34536246.
- ^ Catteruccia M, Verrigni D, Martinelli D, Torraco A, Agovino T, Bonafé L, et al. (March 2014). "Persistent pulmonary arterial hypertension in the newborn (PPHN): a frequent manifestation of TMEM70 defective patients". Molecular Genetics and Metabolism. 111 (3): 353–359. doi:10.1016/j.ymgme.2014.01.001. PMID 24485043.
- ^ Honzík T, Tesarová M, Mayr JA, Hansíková H, Jesina P, Bodamer O, et al. (April 2010). "Mitochondrial encephalocardio-myopathy with early neonatal onset due to TMEM70 mutation". Archives of Disease in Childhood. 95 (4): 296–301. doi:10.1136/adc.2009.168096. hdl:1854/LU-987464. PMID 20335238. S2CID 2946094.
- ^ Spiegel R, Khayat M, Shalev SA, Horovitz Y, Mandel H, Hershkovitz E, et al. (March 2011). "TMEM70 mutations are a common cause of nuclear encoded ATP synthase assembly defect: further delineation of a new syndrome". Journal of Medical Genetics. 48 (3): 177–82. doi:10.1136/jmg.2010.084608. PMID 21147908. S2CID 8699153.
- ^ IntAct. "TMEM70 interactions". www.ebi.ac.uk. Retrieved 2018-08-16.
Further reading
[edit]- Jonckheere AI, Huigsloot M, Lammens M, Jansen J, van den Heuvel LP, Spiekerkoetter U, von Kleist-Retzow JC, Forkink M, Koopman WJ, Szklarczyk R, Huynen MA, Fransen JA, Smeitink JA, Rodenburg RJ (November 2011). "Restoration of complex V deficiency caused by a novel deletion in the human TMEM70 gene normalizes mitochondrial morphology". Mitochondrion. 11 (6): 954–63. doi:10.1016/j.mito.2011.08.012. PMID 21945727.
- Atay Z, Bereket A, Turan S, Haliloglu B, Memisoglu A, Khayat M, Shalev SA, Spiegel R (February 2013). "A novel homozygous TMEM70 mutation results in congenital cataract and neonatal mitochondrial encephalo-cardiomyopathy". Gene. 515 (1): 197–9. doi:10.1016/j.gene.2012.11.044. PMID 23235116.
- Houstek J, Kmoch S, Zeman J (May 2009). "TMEM70 protein - a novel ancillary factor of mammalian ATP synthase". Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1787 (5): 529–32. doi:10.1016/j.bbabio.2008.11.013. PMID 19103153.
- Talmud PJ, Drenos F, Shah S, Shah T, Palmen J, Verzilli C, et al. (November 2009). "Gene-centric association signals for lipids and apolipoproteins identified via the HumanCVD BeadChip". American Journal of Human Genetics. 85 (5): 628–42. doi:10.1016/j.ajhg.2009.10.014. PMC 2775832. PMID 19913121.
- Karasik D, Hsu YH, Zhou Y, Cupples LA, Kiel DP, Demissie S (July 2010). "Genome-wide pleiotropy of osteoporosis-related phenotypes: the Framingham Study". Journal of Bone and Mineral Research. 25 (7): 1555–63. doi:10.1002/jbmr.38. PMC 3153998. PMID 20200953.
- Davila S, Froeling FE, Tan A, Bonnard C, Boland GJ, Snippe H, Hibberd ML, Seielstad M (April 2010). "New genetic associations detected in a host response study to hepatitis B vaccine". Genes and Immunity. 11 (3): 232–8. doi:10.1038/gene.2010.1. PMID 20237496.
- Bailey SD, Xie C, Do R, Montpetit A, Diaz R, Mohan V, et al. (October 2010). "Variation at the NFATC2 locus increases the risk of thiazolidinedione-induced edema in the Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) study". Diabetes Care. 33 (10): 2250–3. doi:10.2337/dc10-0452. PMC 2945168. PMID 20628086.
- Hendrickson SL, Lautenberger JA, Chinn LW, Malasky M, Sezgin E, Kingsley LA, et al. (September 2010). "Genetic variants in nuclear-encoded mitochondrial genes influence AIDS progression". PLOS ONE. 5 (9): e12862. Bibcode:2010PLoSO...512862H. doi:10.1371/journal.pone.0012862. PMC 2943476. PMID 20877624.
- Cameron JM, Levandovskiy V, Mackay N, Ackerley C, Chitayat D, Raiman J, et al. (January 2011). "Complex V TMEM70 deficiency results in mitochondrial nucleoid disorganization". Mitochondrion. 11 (1): 191–9. doi:10.1016/j.mito.2010.09.008. PMID 20920610.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.