Pantetein
Izgled
| Pantetein | |||
|---|---|---|---|
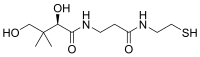
| |||
| IUPAC ime |
| ||
| Identifikacija | |||
| CAS registarski broj | 496-65-1 R | ||
| PubChem[1][2] | 479 439322 R | ||
| ChemSpider[3] | 466 | ||
| EINECS broj | |||
| KEGG[4] | |||
| MeSH | |||
| ChEBI | 16753 | ||
| Bajlštajn | 1714196 R | ||
| 3DMet | B00185 | ||
| Jmol-3D slike | Slika 1 Slika 2 | ||
| |||
| |||
| Svojstva | |||
| Molekulska formula | C11H22N2O4S | ||
| Molarna masa | 278.37 g mol−1 | ||
| Srodna jedinjenja | |||
| Srodna jedinjenja | |||
|
Ukoliko nije drugačije napomenuto, podaci se odnose na standardno stanje (25 °C, 100 kPa) materijala | |||
| Infobox references | |||
Pantetein je cistaminski amidni analog pantoteinske kiseline (vitamina B5). Dimer ovog jedinjenja, pantetin je široko poznat, i smatra se potentnijim oblikom vitamina B5 od pantotenske kiseline. Pantetein je intermedijar u produkciji koenzima A u telu.[5]
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast 17 (1): 48–55. DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑ Suriender Kumar, G.T. Phillips, John W. Porter (February 1972). „Comparative biochemistry of fatty acid synthesizing enzyme systems: A review”. International Journal of Biochemistry 3: 15–32.
